Economics of Innovation
Optimal innovation balances willingness and ability to pay with the benefits of future R&D. Our wide-ranging programme aims to promote innovation in health care, increase our understanding of its value, and encourage R&D. New models of innovation should streamline development, reduce costs, and speed patient benefit.

Would Waiving COVID-19 Vaccines Patents Save Lives?
18 May 2021
This blog discusses the rationale behind a COVID-19 patent waiver decision and contributes to the current debate by producing a balanced view on the benefits and…
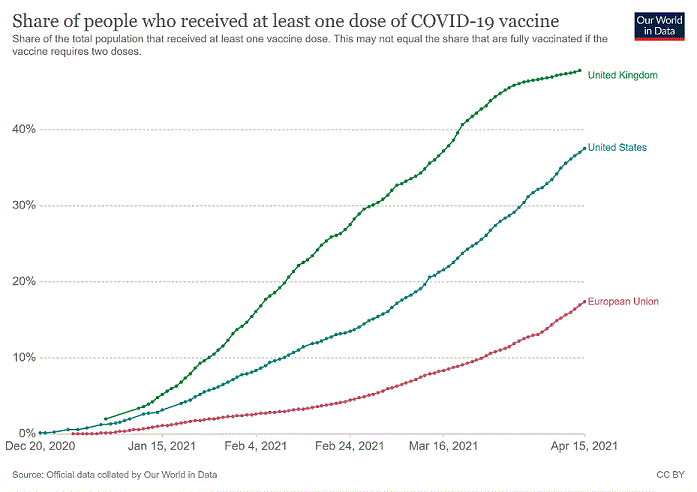
Key Learnings From COVID-19: The Importance of Portfolio Management and How to Procure, Pay For, Distribute, and Use Vaccines During a Pandemic
21 April 2021
In this blog, we reflect on some of the key learnings from the recent pull and push strategies to develop COVID-19 vaccines in the UK, the…

Time to Tackle the Challenges around Combination Therapies for Cancer
29 March 2021
A new Editorial reviews three solutions to the price and value challenge to reimbursing combination products. Higher thresholds are not justifiable. Evidence to support use of…

Does Government Funding Increase Public Sector Development of New Medicines?
5 March 2021
A new paper, published last week in Applied Economics, by Dimitrios Kourouklis, Senior Economist at OHE, looks at the question of whether government funding increases public…

It Takes Two to Tango: When do Conditional Reimbursement Risk-Sharing Schemes Work for Both Parties?
1 December 2021
Faster regulatory approval has not necessarily achieved faster patient access. A new OHE paper sets out how risk-sharing can tackle this, enabling resolution of differences of…
Payment Models for Multi-Indication Therapies
1 November 2021
For the growing number of multi-indication medicines, access may be delayed or even denied due to challenges in linking payment with a medicine’s value across those…

Working Towards a Sustainable, Healthy Market for Vaccines: a Comprehensive Framework to Support Policy Dialogue and Decision-Making
1 September 2021
By placing a strain on health care systems and the global economy, the COVID-19 pandemic clearly shows the need to more comprehensively understand both supply- and…
The Lower Drug Costs Now Act and Pharmaceutical Innovation
1 September 2021
The US spends significantly more on healthcare per person than other wealthy countries. H.R. 3 is a recent policy proposal aimed at reducing national spending on…

Bridging the Gap: Pathways for Regulatory and Health Technology Assessment of Histology Independent Cancer Treatments
8 January 2021
Histology independent therapies are changing the picture of cancer treatment and in so doing don’t ‘fit the frame’ of value assessment. What are the key challenges…