Drug Development/R&D
New medicines lead to improved patient outcomes and societal benefits, but R&D is a long and costly process. Ongoing health economics research aims to find the right balance between incentivising manufacturers to innovate and ensuring affordable access for health systems.

Does Government Funding Increase Public Sector Development of New Medicines?
5 March 2021
A new paper, published last week in Applied Economics, by Dimitrios Kourouklis, Senior Economist at OHE, looks at the question of whether government funding increases public…
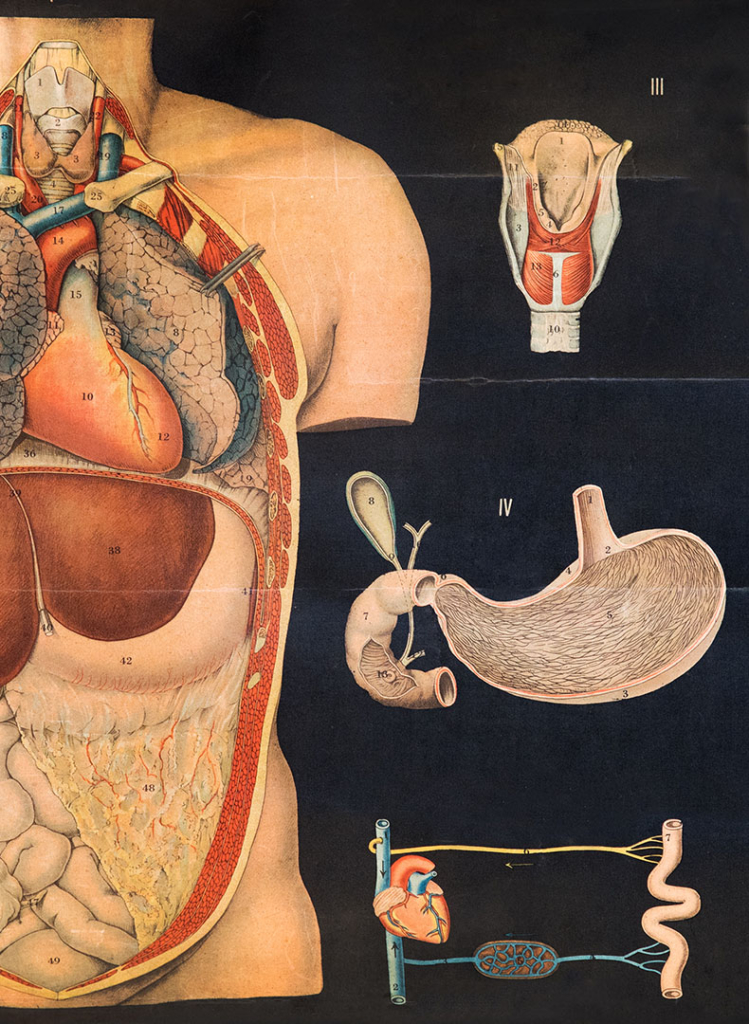
Payment Models for Multi-Indication Therapies
1 November 2021
For the growing number of multi-indication medicines, access may be delayed or even denied due to challenges in linking payment with a medicine’s value across those…

OHE Authors Develop a Supply and Demand Model of Pharmaceutical Markets to Set Cost-Effectiveness Thresholds to Maximise and Distribute Social Welfare
15 June 2020
OHE authors develop a supply and demand model of pharmaceutical markets to analyse the social welfare distribution between consumers (payers) and developers (industry) to set an…
How Should the World Pay for a COVID-19 Vaccine?
29 April 2020
Prof. Adrian Towse discusses the options for funding the development and manufacture of a vaccine, reflecting on their strengths and weaknesses, considering what may happen with…

Financing and Scaling Innovation for the COVID Fight: A Closer Look at Demand-Side Incentives for a Vaccine
1 April 2020
A COVID-19 vaccine is needed now, but timelines (12-18 months) create large market risk. By the time a vaccine is ready, the crisis may have passed.…

How do we Get and Pay for New Antibiotics? Proposed New Models for Value Assessment and Contracting for Payment.
26 March 2020
This presentation to the Australian Society for Antimicrobials (ASA) meeting in Melbourne, on 27th February 2020 draws on OHE research, funded by the Wellcome Trust, on…

Are Cost-Effectiveness Thresholds Fit for Purpose for Real-World Decision Making on Access to and Pricing of Pharmaceuticals? A New OHE Paper on Current Methods and Practices, and a Novel Approach for Optimal Thresholds
3 March 2020
OHE has published a white paper discussing the relative merits and shortfalls of current approaches to defining, estimating, and applying cost-effectiveness thresholds in HTA. This will…

A Final MVAC Blueprint—and the Start of an R&D Revolution?
6 February 2020
TB kills 1.6m people annually – the world’s deadliest infectious disease. The Market-Driven, Value-Based Advance Commitment (MVAC), creates and guarantees a market for better TB treatment.…

Data Governance Arrangements for Real-world Evidence in Japan
12 September 2019
A new OHE Consulting Report describes the current status of real-world data in Japan, including core legislation and governance arrangements. The authors sought to understand how…