In this report, we explore the potential impact of the National Institute of Health and Care Excellence (NICE) decision outcomes on healthcare decision-making in 12 countries across the globe (Australia, Brazil, Canada, France, Italy, Israel, Japan, South Korea, Poland, Saudi Arabia, Sweden, and the United Arab Emirates). We used quantitative and qualitative methods to estimate the level of impact and potential underlying mechanisms.
Key takeaways
- Our research suggests that NICE decisions have some degree of impact on healthcare decision-making in most of the countries considered.
- However, the content considered, the circumstances when considered, and the extent to which NICE decisions have an influence vary significantly.
- Negative NICE decisions are likely to have more impact internationally compared to positive decisions. Local decision-makers are potentially more inclined to scrutinise the submitted evidence, or in price setting, using the negative decision as leverage to negotiate a lower price.
- That being said, the future impact of NICE on the global Health Technology Assessment (HTA) landscape may change in the near future, due to the adoption of the European regulation on HTA and the increase in collaborations among HTA agencies.
HTA is a crucial component of health care decision-making in many countries with wide-reaching consequences for population health. Within the global HTA landscape, the National Institute for Health and Care Excellence (NICE) plays a prominent role. However, there is limited evidence on the extent to which this is the case and, if it is, how NICE’s decision outcomes on individual interventions affect HTA in other countries.
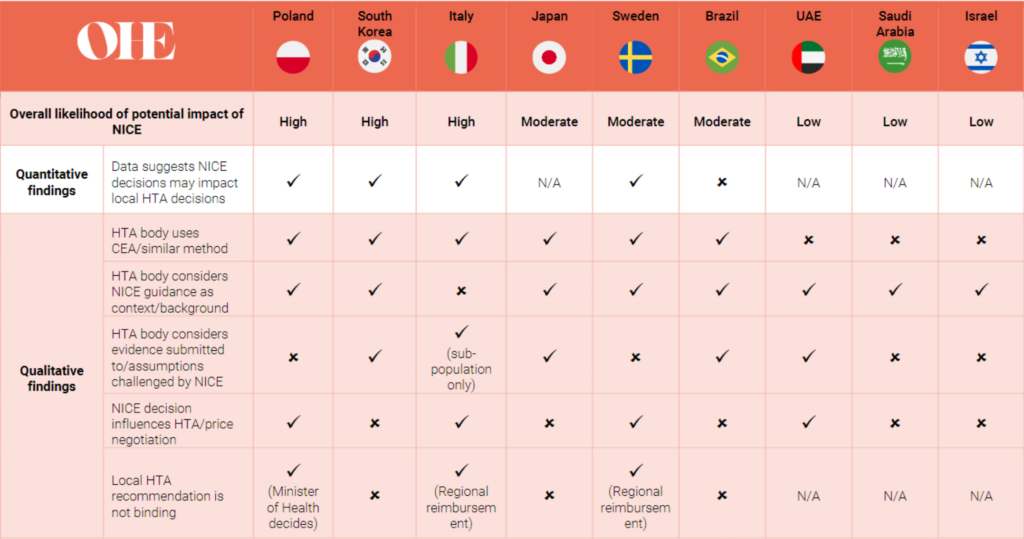
N/A: No data available
Figure 1: Summary table of the potential international impact of NICE decisions through quantitative and qualitative research
We collected a dataset of HTA decision outcomes made by NICE between January 2018 to December 2019 and the equivalent decisions made by eight international HTA bodies to estimate the impact of NICE. To complement this work, we conducted interviews with industry, relevant decision makers and academics, some of which were included in the quantitative analysis and some additional countries where data collection was not possible.
We conclude that NICE guidance is likely to have some impact in other countries, but this varied from being included as context or background for decision-makers, providing information on assumptions in the economic model to be scrutinised or that can impact the price negotiations. We also surmise that indirect mechanisms for influence are likely to exist, for example, similar methodologies (i.e., cost-effectiveness analysis), transparency and availability of decisions online, and the reputation of NICE and UK universities.
Although it is too early to predict outcome of increased collaboration between HTA agencies, they might strengthen NICE’s role on the international stage. On the other hand, given that NICE is not included in European activities to streamline HTA (such as Joint Clinical Assessment), this and other post-Brexit activities could weaken the influence of NICE, particularly in Europe.
This consulting report, ‘NICE enough? Do NICE’s Decision Outcomes Impact International HTA Decision-making?’, was commissioned and funded by Sanofi UK.





