The value of antibiotics often extends beyond what HTA captures. The STEDI framework aims to capture that broader value and our report outlines a roadmap to realising its full potential.
Key Takeaways
- Current value assessment of antibiotics cannot address the market failures that hinder antibiotic innovation.
- The STEDI framework – Spectrum, Transmission, Enablement, Diversity, and Insurance – offers a comprehensive approach to capturing the broader value of antibiotics.
- Challenges with implementing the STEDI framework stem from the uncertainty and the complexity underlying the evaluation, but can be overcome.
- Our roadmap provides a route to appropriate quantitative value assessment of antibiotics in the long run, via progression through three phases.
- Proposed actions require collective, interdisciplinary action, and significant progress can be made with meaningful stakeholder collaboration.
As one of the backbones of modern medicine, antibiotics are a critical component of healthcare, resolving infection and limiting its spread whilst also ensuring that medical procedures, including chemotherapy and surgery, can continue and advance. Despite their vital role, their true value often extends beyond what is captured during traditional Health Technology Assessment (HTA).
The STEDI framework, encompassing five value elements – Spectrum, Transmission, Enablement, Diversity, and Insurance – offers a comprehensive approach to capturing the broader value of antibiotics. However, challenges and uncertainties affect its successful application.
This report outlines a research roadmap to overcome these hurdles in three phases. Ultimately, it paves the way toward a fully integrated assessment of the STEDI framework based on the widely established HTA approach to capture the outcomes in quality-adjusted life years (QALYs) in the long run.
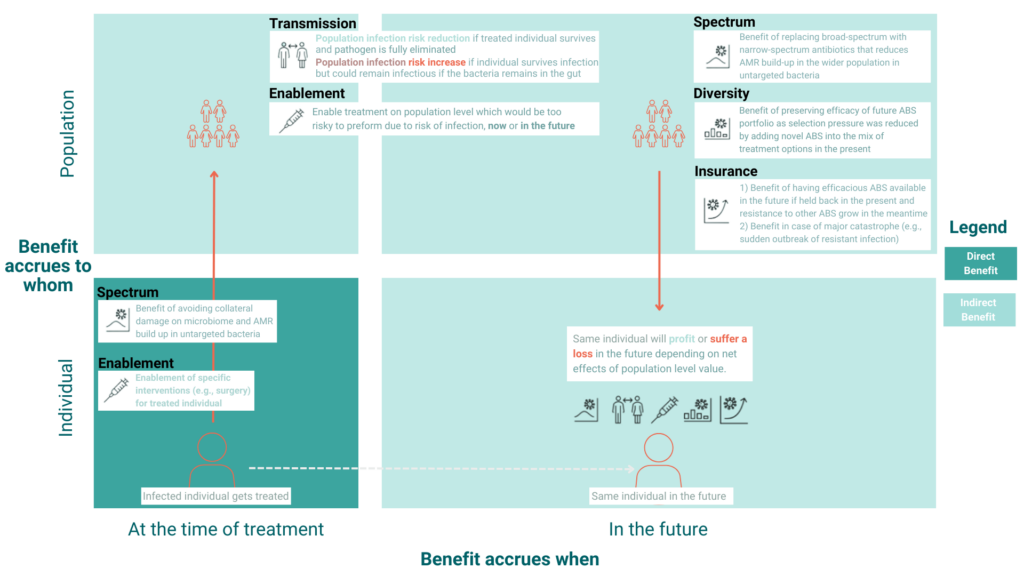
STEDI is crucial for setting the right incentive for an active antibiotic pipeline, but substantial sources of uncertainty hinder its successful application to date. An antibiotic’s broader value can accrue to the treated individual or the wider population and accumulate during treatment or further into the future. Thus, when conceptualising the different value elements, it may be helpful to consider to whom the benefits accrue and when they accrue.
Figure 1 visualises these distinct benefits by separating the direct benefit (benefit to the individual treated at the time of treatment) from the indirect benefit (externality to a wider population or future benefit to the individual). Only a few STEDI elements have a direct benefit, but population effects today and in the future feedback to the treated individual later in life assuming their survival. In addition, some elements have multiple mechanisms (e.g., spectrum value), making their conceptual separation even more challenging.
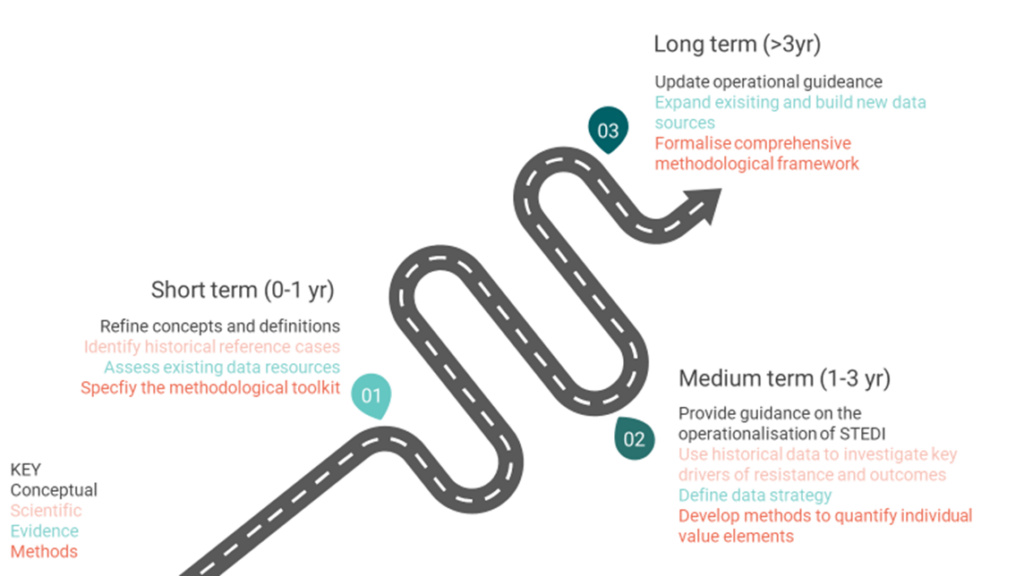
To fully realise the potential of the STEDI framework, the report suggests a research roadmap in three phases:
- Achieve clarity and consensus on underlying definitions and concepts of each STEDI element in the short term.
- Progress science and develop pragmatic methods to quantify each element in the short term.
- Set standards for data collection, generate relevant evidence and establish advanced methodological guidance to ensure a fully integrated QALY-based quantification of the STEDI framework in the long
The report was commissioned and funded by The Association of the British Pharmaceutical Industry (ABPI).





