Drug Development/R&D
New medicines lead to improved patient outcomes and societal benefits, but R&D is a long and costly process. Ongoing health economics research aims to find the right balance between incentivising manufacturers to innovate and ensuring affordable access for health systems.

‘Macro’ Evaluation of the NIHR Oxford Biomedical Research Centre
1 May 2017
The Oxford Biomedical Research Centre (BRC) was established in April 2007. OHE and RAND Europe were commissioned by the Oxford BRC to undertake a programme of…

OHE at AES 2016: Early Access Schemes, Equity Adjusted QALYs, Multi-indication Pricing and Formulary Development
2 August 2016
The Spanish Health Economics Association (AES) Conference was held at the University of Murcia, 15-18th June 2016. The topic of the meeting was “Reforms under the…

OHE Workshop: Surrogate Endpoints as Predictors of Overall Survival in Oncology
14 July 2016
On Tuesday 28th June 2016 OHE held a workshop on the topic of surrogate endpoints as predictors of overall survival in oncology. The workshop was commissioned…

What Drives Variations in Relative Effectiveness Across Countries?
31 May 2016
This study provides an analytical framework, drawing on production function theory, to identify and quantify the determinants of relative effectiveness and sources of variation in the…

Spotlight on OHE: Beyond QALYs, Managed Entry Agreements, Business Models for Antibiotics, and the UK Market for Medicines.
12 April 2016
The blog provides links to slide sets for presentations relating to going ‘beyond QALYs’ when valuing health care interventions, managed entry agreements within the UK pharmaceutical…

New Publication: Spillovers between Public and Private Sector Biomedical and Health Research
8 March 2016
OHE’s Yan Feng and Jorge Mestre-Ferrandiz have co-authored a new publication on quantifying the spillovers between public and private sector biomedical and health research and development…

New Publication: Headroom Approach to Device Development
23 February 2016
OHE’s Amanda Cole co-authors a new publication on the headroom approach, which can help assess the commercial value and viability of medical device development.
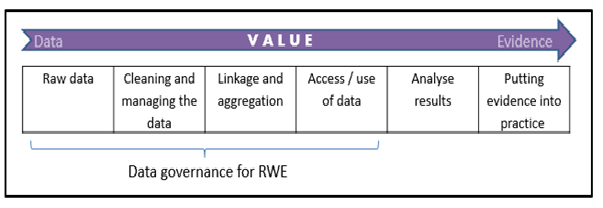
How to Manage Data Governance in an Era of Real World Evidence (RWE): Working Towards a Set of International Standards
24 November 2015
A new OHE Consulting Report assesses information governance arrangements for real-world data in eight countries, and makes recommendations towards an ideal governance framework.

Newly Published Analysis: Dementia: the R&D Landscape
16 November 2015
Just published by the World Dementia Council and OHE is an analysis which sets out the dementia R&D landscape. This analysis was undertaken by OHE for…