The many changes in Health Technology Assessment (HTA) methods and processes over time, are a challenge to navigate let alone stay abreast of. This report explores the breadth of variation in past and current positioning of HTA agencies in 14 countries, on five key methodological topics: discount rates, modifiers, patient involvement, real-world evidence (RWE), and surrogate endpoints.
Key Takeaways
- HTA agencies’ guide to methods have become more detailed over time. However, some HTA agencies have not yet introduced explicit guidelines on all the considered topics, such as patient involvement, RWE and surrogate endpoints.
- The shifts in the position of the HTA agencies vis-à-vis the five key topics show increasing adaptability and pragmatism in evaluating new treatments, with variation among agencies.
- Methods guidelines remain heterogenous, highlighting a lack of harmonisation among HTA agencies leading to variations in evidence requirements from different stakeholders.
- International collaborations represent a useful route to accelerate HTA reforms. These alliances could facilitate timely and consistent changes in HTA methods, which could benefit all stakeholders and improve outcomes for patients.
We investigated 14 HTA agencies across four continents, as described below.
Data obtained from our literature search and expert interviews was used to pinpoint the factors propelling reforms in M&P. These drivers were then classified into three overarching themes: stakeholders, country-specific context, and cross-border context.
Below we provide a summary of our findings for the topic concerning discount rates. Similar analyses were performed for the other four remaining topics and are presented in the main report. The general trend over time across all HTA agencies leans towards using lower discount rates, which reflects a greater degree of value placed on future outcomes.
Figure 1 shows the agencies’ current positioning on discount rates. We found that discount rates for the HTA agencies included in this study are spread over a range from 1.5% to 5%, with the majority of HTA agencies using a rate between 2.5% and 3.5%. Most HTA agencies have the same discount rate across costs, outcomes, and time horizons. However, some of them have different discount rates for costs and benefits, and for others, the discount rates decline with a longer time horizon.
Figure 1 HTA agency positions – Discount rates
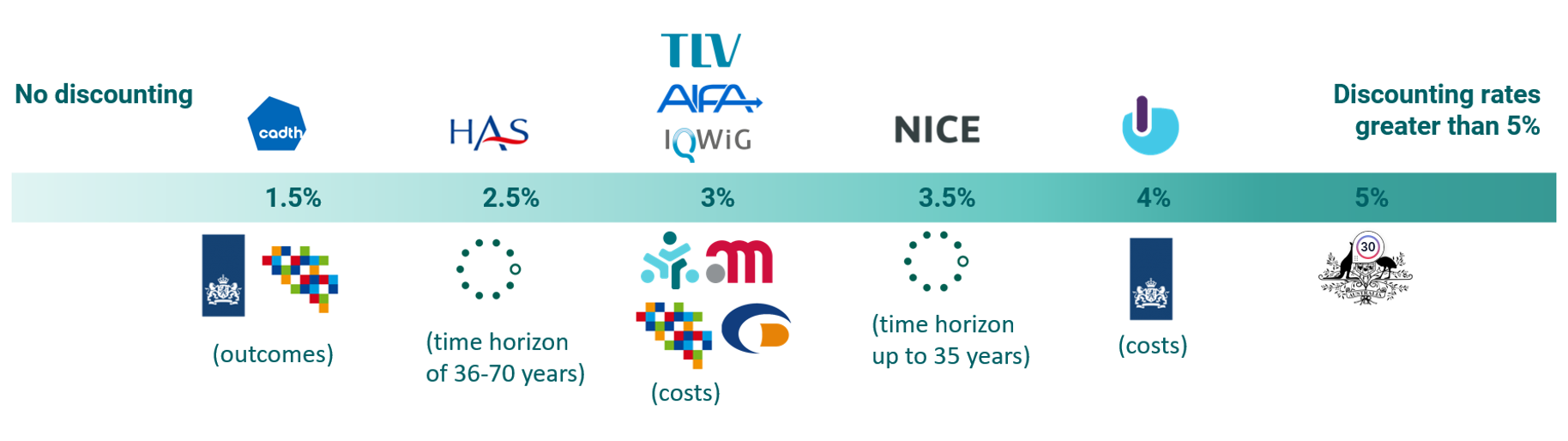
Figure 2 presents a dynamic heatmap created to showcase the evolution of the positioning for each agency over time. HTA agencies in five of the 14 countries (ACE, AIFA, AEMPS, DMC and ZIN) have explicitly adopted a discount rate since 2010, and four of the nine HTA agencies which had already specified their base-case discount rate by 2010 (CADTH, CDE, HAS, and INFARMED) have changed it since 2010.
Figure 2 Dynamic heatmap – Discount rates
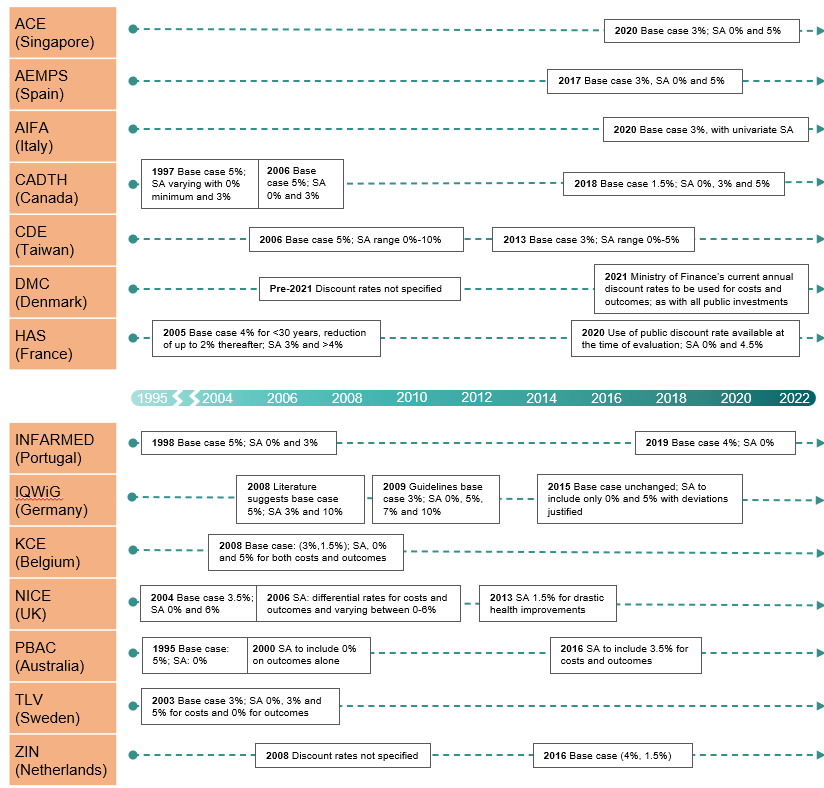
The timeline in figure 3 summarises the changes highlighted in the dynamic heatmap above, providing nuance to the variation in positioning over time. Reasons for reform to the discount rate were generally motivated by two main drivers: HTA practice in other countries and the country-specific healthcare policy, political and legal context (e.g., DMC and HAS adjusting discount rates in line with other public sector investments).
Figure 3 Evolution of HTA agency positions over time – Discount rate
General trends showed that HTA agencies’ positions with regards to the topics investigated have become clearer over time. This was generally achieved by introducing new guidelines on specific topics, clarifying existing ones, or publishing additional separate documents explaining agencies’ positions. Furthermore, the direction of change is generally oriented towards more flexibility and pragmatism in evidence acceptance.
However, some HTA agencies have not yet introduced explicit guidelines on some topics, which has the potential to increase the difficulty of navigating evidence requirements for manufacturers and prolong submission times. The current heterogeneity among countries’ guidelines highlights the lack of international harmonisation of HTA guidelines and the presence of national barriers to the introduction of new reforms and methodological changes.
International collaborations represent a useful route to accelerate changes, particularly for those agencies with limited or no guidance in certain topics. However, the extent to which potential reforms can be implemented depends on attitudes towards change within each country.
‘Navigating Change: How Have HTA Agencies Evolved Their Methods Over Time?’, was commissioned and funded by Merck Sharp & Dohme, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA’






