Unlocking the Value of Combination Therapies

The goal of pricing of pharmaceutical innovations is to ensure that patients can access medicines in a way that is sustainable for healthcare systems whilst also supporting a sustainable stream of innovation that delivers continuous improvements in the treatment options available for patients. Prices send signals to innovators about where to focus their R&D efforts, as well as determine the overall level of investment in health and expected value of innovation in the pipeline.
This is why EFPIA is working with governments and stakeholders to identify and promote pricing approaches that deliver a ‘triple win’: ensure medicines are accessible, the healthcare system is sustainable, and the innovation pipeline is robust.
In this report, we investigate the theory and practice of value-based pricing in the pharmaceuticals industry, and provide recommendations for how implementation of value-based pricing can be enhanced to deliver the triple win of patient access; healthcare system sustainability; and innovation.
Summary of recommendations
Key findings

Figure 1 illustrates which elements of value are considered in health technology assessment processes by selected European Union member states. The value elements are ranked in order of frequency of consideration. We find that are some value elements which all countries theoretically consider, although there may be variation in if they are considered in practice. In addition, all countries make some provision for capturing broader dimensions of value to society. However, some value elements are rarely considered in value assessment. The least recognised value elements are innovation, the value of treating rare diseases, improvements to the process of care, and indirect medical costs.
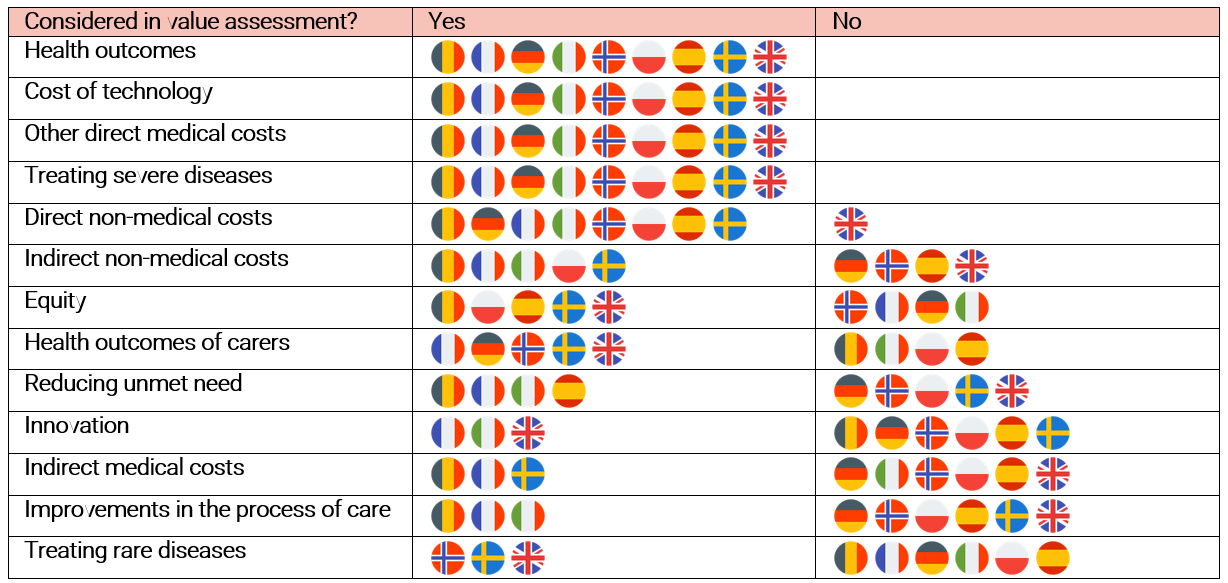
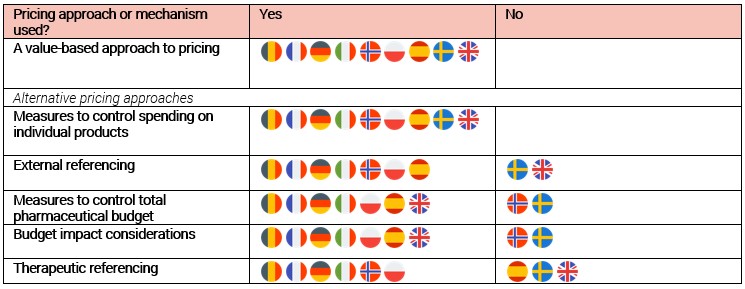
Figure 2 shows which pricing approaches and price control measures are used by selected European Union member states. The approaches are ranked by frequency of consideration. We find that all of these countries make some provision to reflect the results of value assessment in their pricing and reimbursement decisions. There may be a clear, mechanistic link, as in the case of countries using explicit cost-effectiveness thresholds, or a more deliberative and/or qualitative approach may be used. However, all countries considered in our research also make use of other pricing approaches or price control measures that disrupt the alignment between value and price. The most prevalent of these are external reference pricing and measures to control overall pharmaceutical expenditure, both of which are used in seven of nine countries.
Delivering the Triple Win: A Value-Based Approach to Pricing


