OHE convened a roundtable discussion to present and discuss recent work by OHE colleagues and collaborators on value attribution frameworks for combination therapies.
The Policy Context
An increasingly common strategy for treating many cancers is to use medicines in combination or in close sequence. However, valuing and pricing the components of a combination therapy (CT) gives rise to several challenges for payers, Health Technology Assessment (HTA) bodies, industry, and health systems. This leads to delays in or lack of access to valuable therapies and more unintended consequences in the long term.
Why We Need a New Outcomes-Based Value Attribution Framework for Combination Regimens in Oncology, co-authored by Adrian Towse (OHE), Dr Mickael Lothgren (Ipsen), Dr Lotte Steuten (OHE) and Andrew Bruce (Amgen), explains the ‘not cost-effective at zero price’ paradox: when an ‘add-on’ medicine is used in conjunction with a ‘backbone’ medicine to form a CT which is clinically superior to either medicine delivered as a monotherapy, the ‘add-on’ medicine might not be cost-effective at any nonnegative price when the combined regimen increases treatment duration. In such circumstances, CTs may not receive reimbursement and patients do not receive the most appropriate care. Even when CTs are reimbursed, a challenge arises of how to attribute value between products in a way that incentivises innovation in the future. There is no agreed way for manufacturers of medicines that together form a CT to split proceeds of the sale of the combined regimen (assuming different companies produce the components), which may lead firms to underinvest in developing these types of treatments.
To make the situation even more challenging, any solution must also be workable within current Health Technology Assessment (HTA) systems to be operationalised in practice. OHE has been working with Mickael Lothgren (Ipsen) and Andrew Bruce (Amgen) on a General Outcome-based Value Attribution Framework for Combination Therapies, presented in a recently published paper.
The Roundtable
The roundtable, hosted by OHE and sponsored by Amgen, was held to foster a constructive discussion on solutions proposed in the context of the current methodological and policy debate. It was held on 6th December 2022, bringing together over 50 delegates (both online and in-person) from industry and academia with strong health economic expertise. Ten different countries were represented, including Australia, the UK and other major European nations.
Gavin Lewis, VP of Global Value and Access at Amgen, discussed the four policy domains that need to be addressed to improve access to CTs: the incentive problem, the implementation problem, competition law, and the value attribution problem. The solution proposed by Towse & Lothgren et al. addresses the last of these – the value attribution problem – and does so while satisfying three desirable properties; namely that the framework was universal (applicable to all possible CT configurations), logical and symmetric (does not depend on the order in which component treatments were launched), and complete (all value is apportioned to the constituent therapies).
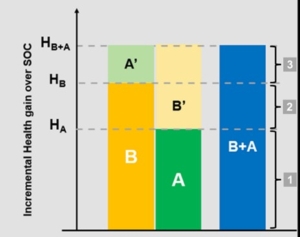
The co-authors presented challenges surrounding attributing value to CTs and the proposed outcome-based value attribution framework. The new solution apportions value based on a weighting formula that incorporates the effectiveness of the CT as a whole and that of each component medicine used as a monotherapy. For example, in the figure above, the value is attributed to the CT components ‘B+A’ as follows: the value of segments 1 and 3 are split equally between A and B, while the value in segment 2 accrues entirely to B. This ensures that the treatment with the better monotherapy effectiveness (in this case, B) receives a higher share of the value of the combination.
Note that this solution is straightforward under a ‘ full outcomes information scenario’, i.e., when information about the monotherapy effectiveness of both the backbone and add-on therapies is known. However, Towse & Lothgren et al. make two suggestions to handle a ‘partial outcomes information scenario’ where the effectiveness of the add-on as monotherapy is unknown: a Bayesian approach to estimate expected outcomes (using imputation and expert elicitation) and Value of Information Analysis to estimate whether additional evidence should be generated to improve estimates of the value of monotherapies.
Previously, another group of experts developed a solution to the value attribution problem, presented in Briggs et al. (2021). This proposal includes the partial outcomes information incremental value approach and the full information monotherapy ratio approach. However, these only satisfy all of the previously mentioned desirable properties (universality, logic & symmetry, completeness) under a limited set of assumptions that are not expected to hold in practice. Dr Andrew Briggs presented his approach and noted areas of convergence with the OHE framework. He argued that the OHE approach’s principal advantage is that it is contained within a single attribution framework and that its principal disadvantage is that it is more complex and less intuitive in his view.
Expert panellists discussed the properties of the solution proposed by Towse & Lothgren et al. and compared the framework to those proposed by Briggs et al. It was noted that the incremental value approach presented by Briggs does not depend on information about the monotherapy effectiveness of the add-on therapy, but concerns were raised that this approach may not provide efficient incentives to develop new treatments. For example, because the incremental value approach is not symmetric, perverse incentives could be created for companies to delay market access if the expected reimbursement from being an ‘add-on’ exceeds that of being a ‘backbone’, unnecessarily delaying patient access to treatment.
Beyond the relative merits of different proposals, there was consensus about general challenges going forward:
- The method assumes knowledge of the effectiveness of each component therapy when administered as a monotherapy – an assumption that all participants recognised as not reflecting the realities of many CTs. Panellists agreed that one way forward is to impute estimates for the effectiveness of the monotherapy, as this would avoid wasteful data collection efforts to assess the effectiveness of monotherapies, which will never be used as such and are likely to provide inferior clinical benefit.
- Participants also noted the need for a framework to inform price negotiations between manufacturers of a CT. It was noted that there is a need for inter-stakeholder collaboration between HTAs, companies, and competition regulators to agree on a framework which facilitates productive negotiations.
- There is a need for further research on handling the uncertainty of treatment effectiveness within the framework. Because the value attribution framework applies to on-patent (hence often new-to-market) medicines, there may be insufficient evidence of their effects on real-world patients to precisely estimate their value. Methods to address these complexities are available but must be workable in existing HTA frameworks.
Next Steps
Panellists emphasised the broad areas of alignment between proposed solutions and commented on the importance of providing multiple options to HTA, payers and other stakeholders in different countries and jurisdictions to find the most suitable for each context. Key conclusions include:
- There is awareness of the problems related to the adoption of CTs overall, but solutions so far have been sub-optimal or partial. Published proposals and the roundtable demonstrated that a general approach for apportioning the value among CT components is possible, and options are available; the involvement of HTA and the wider healthcare community is crucial in agreeing and implementing them.
- More work on the other three policy domains (the implementation problem, incentive problem, and competition law) is needed for the potential of this framework to be fully realised.
- The next steps include more work around the partial information scenario as well as the application of the new framework using case studies and/or a pilot. Case studies can help identify the most recurrent CT typologies so far and those likely to enter the market. Because of the challenges around valuing and pricing CTs, it is important to create a framework that can both provide patients access to the best treatments and create efficient signals to the industry to innovate. Now is the time for stakeholder collaboration to ensure these pioneering contributions make a positive real-world impact on patient access and innovation going forward.
Related research
Towse, A., Lothgren, M., Steuten, L. and Bruce, A., 2022. Why We Need a New Outcomes-Based Value Attribution Framework for Combination Regimens in Oncology. Value in Health, 25(11), pp.1821–1827. 10.1016/j.jval.2022.06.009.
Towse A., Lothgren M., Bruce A, & Steuten L. (2022) Proposal for a General Outcome-based Value Attribution Framework for Combination Therapies. OHE Consulting Report. Available from https://www.ohe.org/publications/proposal-general-outcome-based-value-attribution-framework-combination-therapies.