Dr Steven Pearson has convened a US policy workgroup of payers, drug and device manufacturers, patients’ groups, and clinician specialty societies to help develop a “value…
Dr Steven Pearson has convened a US policy workgroup of payers, drug and device manufacturers, patients’ groups, and clinician specialty societies to help develop a “value framework”.
Payers in the US do not embrace a single, dominant method for determining the value of new technologies. With this mind, Dr Steven Pearson has convened a US policy workgroup of payers, drug and device manufacturers, patients’ groups, and clinician specialty societies to help develop a “value framework” to guide the assessment of drugs and devices in the US. In this Briefing, based on an OHE lunchtime seminar, he presents an early draft version of this framework as a method for integrating elements of cost-effectiveness and affordability.
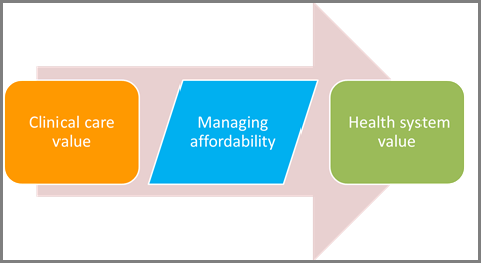
The draft framework splits the conceptual idea of “value” into two components, clinical care value and health system value. Judgements as to the clinical care value of drugs and devices are based on five elements: comparative clinical effectiveness; additional benefits; contextual considerations; and incremental cost per outcomes achieved. Once clinical care value has been established, payers and manufacturers can work together to manage affordability and thus obtain health system value (see Figure 1).
Figure 1. Elements in a payer assessment of value
Copyright ICER 2014
In this
Briefing, Dr Pearson uses conceptual and real-world examples to demonstrate how the framework can be used by payers to determine value ratings for new health care technologies. He summarises the similarities between this framework and the methods used by the National Institute for Health and Care Excellence (NICE) in the UK, before concluding with his thoughts on bridging the gap between cost-effectiveness and affordability in the US.
Dr Pearson is the Founder and President of the Institute for Clinical and Economic Review (ICER), an independent non-profit health technology assessment organization based in Boston, Massachusetts. He is a Lecturer in the Department of Population Medicine at Harvard Medical School and has served as Special Advisor, Technology and Coverage Policy, within the Coverage and Analysis Group at the US Centers for Medicare and Medicaid Services. He has also been a Senior Visiting Fellow at England’s National Institute for Health and Care Excellence (NICE), the Vice Chair of the US Medicare Evidence Development and Coverage Advisory Committee (MedCAC), and a Senior Fellow at America’s Health Insurance Plans (AHIP).