The OHE model varies from other models of biosimilar markets evolution by starting with transaction prices, rather than list prices, and factoring in the collection of…
The OHE model varies from other models of biosimilar markets evolution by starting with transaction prices, rather than list prices, and factoring in the collection of ‘Patient Safety Year’ (PSY) data. These data will capture actual experience with a biosimilar, increasing the comfort level of prescribers and encouraging a gradual increase in market share. PSY data also have important implications for payers and governments, whose options also were outlined in this presentation.
This is the fourth on our series of posts based on the OHE seminar and summarises the remarks of Prof Adrian Towse and Dr Jorge Mestre-Ferrandiz.
Describing and/or forecasting the market for biosimilars must first recognize that these products are not identical, i.e., not ‘biogenerics.’ This affects both the time required for effective market penetration and constrains what payers can reasonably do to realise savings. Perhaps most importantly, biosimilars may raise concerns among prescribers, payers and consumers about the extent of interchangeability with the originator product and among competing biosimilars. Safety is highlighted as a concern more often than efficacy.
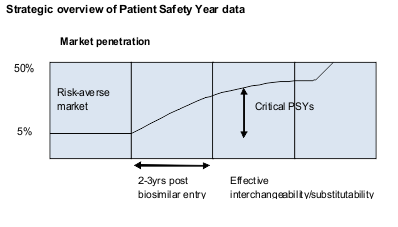
According to Towse and Mestre-Ferrandiz, the centrality of safety concerns dictates the collection of post-launch ‘Patient Safety Year’ (PSY) data. Payers, biosimilars manufacturers and regulators all stand to benefit. Assuming that the PSY data show interchangeability for particular products, clinicians should be more willing to prescribe biosimilars in those therapeutic areas within two to three years after introduction, they estimate. Marketing penetration, expected to be below 5% of market share initially, then should begin to rise as competition is based more on price.
The OHE findings on price trajectories and price discounting strategies vary from other models by starting with transaction price, not list price, and recognising the critical role of PSY data. In particular, the OHE model (1) predicts less rapid erosion of price in the short term and (2) suggests lower prices than some other models in the medium term because PSY data can both help simplify the regulatory process for subsequent entrants and reduce prescriber hesitancy.
The absence of PSY data that support interchangeability will affect pricing decisions. Until they are available, deep discounting by biosimilar manufacturers will not necessarily speed market penetration. For originators, lowering price to preserve market share could slow biosimilar market penetration and, so, the collection of PSY data on the biosimilar.
Payers’ options also are constrained, both by the lack of PSY data and by the fact that much of the market is hospital based. Uncertainty about differences makes it unwise to encourage pharmacy substitution immediately on biosimlar entry; this would affect only a small portion of biologics use anyhow. Uncertainty about safety also complicates direct price intervention, through reference pricing, for example, because physicians still may be hesitant about using the biosimilar. Direct price intervention in such a tentative market is likely to discourage entry in any case by reducing potential profits. In short, Towse and Mestre-Ferrandiz ague, it is not possible to jump start a biosimilars market by forcing down prices or imposing substitutability.
The best option for payers and governments is a ‘market support’ policy that includes the infrastructure investment needed for both monitoring outcomes and collecting PSY and other pharmacovigilance data. Collecting high quality data can both minimise adverse events by better defining any safety risks and help demonstrate the value for money offered by biosimilars.
Prof Towse is Director of the OHE and Dr Mestre-Ferrandiz is Senior Economist at the OHE.
Publication now available for download: Mattison, N., Mestre-Ferrandiz, J. and Towse, A. eds (2010) Biosimilars: How much entry and price competition will result? London: Office of Health Economics.
Download a comprehensive explanation of the OHE model here.