Health Technology Assessment (HTA)
Health Technology Assessment (HTA) is a multidisciplinary process that uses explicit methods to determine a health technology’s value for healthcare decision-making. Attitudes towards HTA vary globally, with some countries using a variety of methods and analyses and others hardly any.

New Publication: Headroom Approach to Device Development
23 February 2016
OHE’s Amanda Cole co-authors a new publication on the headroom approach, which can help assess the commercial value and viability of medical device development.

New ISPOR Task Force Report on Multiple Criteria Decision Analysis
9 February 2016
Professor Nancy Devlin co-chaired the ISPOR task force on uses of MCDA in health care decision making. The first report from the task force has just…
Extrapolation from Progression-Free Survival to Overall Survival in Oncology
1 December 2016
This Research Paper reports on a literature review of trials using PFS as a surrogate for OS in oncology between 2012 and 2016; a workshop and…

Applying a Multi-criteria Decision Analysis (MCDA) Approach to Elicit Stakeholders’ Preferences in Italy. The Case of Obinutuzumab for Rituximab-Refractory Indolent Non-Hodgkin Lymphoma (iNHL)
1 December 2016
Published paper using MCDA to obtain preferences on decision criteria across three stakeholder groups (patients, clinicians and payers) in Italy and to assess the value of obinutuzumab for rituximab-refractory iNHL.
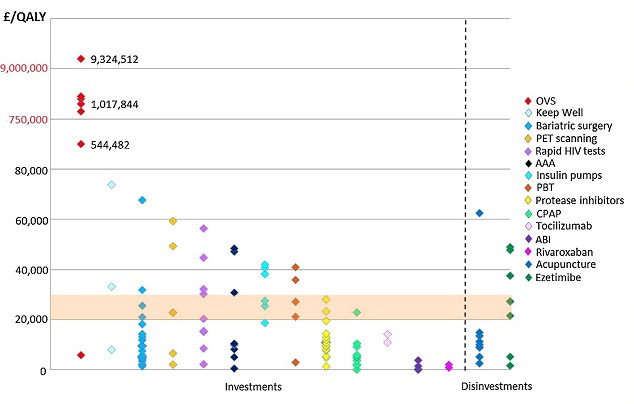
Seminar at Glasgow Caledonian University: Local Health Care Expenditure Plans and Their Opportunity Costs
11 January 2016
OHE’s Sarah Karlsberg Schaffer presented at the Glasgow Health Economics Seminar Series the results of a paper on the value of the cost-effectiveness threshold in Scotland.
Uncertainty and Risk in HTA Decision Making
1 November 2016
The quality of decision-making in key public sector bodies dealing with resource allocation is a major determinant of their efficiency. One of the most difficult and contentious areas of decision-making is the way that uncertainty is dealt with.

How Can Health Technology Assessments in the Asia-Pacific Area Respond to Increased Clinical Uncertainty as a Consequence of Expedited US and EU Regulatory Processes?
8 January 2016
This report provides a detailed summary of a panel session which took place at the HTAi 2016 annual meeting, Tokyo. The panel session was entitled “How…

“New Age” Decision Making in HTA: Is It Applicable in Asia?
1 August 2016
This report provides a detailed summary of a panel session which took place at the HTAi 2016 annual meeting in Tokyo. The panel session was entitled…

A Review of NICE Methods Across Health Technology Assessment Programmes: Differences, Justifications and Implications
1 May 2016
NICE’s decisions exert an influence on the allocation of fixed NHS budgets, but decisions for different types of health interventions (for example drugs and devices) are…