Health Technology Assessment (HTA)
Health Technology Assessment (HTA) is a multidisciplinary process that uses explicit methods to determine a health technology’s value for healthcare decision-making. Attitudes towards HTA vary globally, with some countries using a variety of methods and analyses and others hardly any.

How Important are the Differences Between the EQ-5D-5L and EQ-5D-3L Value Sets?
24 March 2017
There are three EQ-5D value sets available for use in cost effectiveness analysis in the UK and/or England: – the UK EQ-5D-3L value set (often called…

EQ-5D and the EuroQol Group: Past, Present and Future
17 March 2017
OHE’s Professor Nancy Devlin and co-author Richard Brooks have published a new paper that provides an overview of the development of the EQ-5D; the current state…
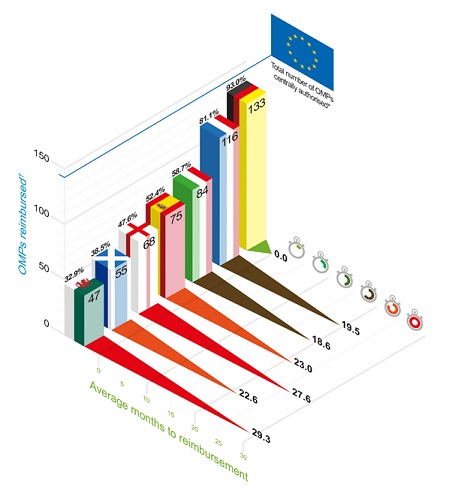
Important Variations in Access to Orphan Drugs in France, Italy, Germany, Spain and the UK
14 March 2017
A new OHE report compares the availability and access of orphan medicinal products (OMPs) in the UK, France, Germany, Italy and Spain.

New Publication: Gene Therapy: Understanding the Science, Assessing the Evidence, and Paying for Value
7 March 2017
Just published is a new report entitled: Gene Therapy: Understanding the Science, Assessing the Evidence, and Paying for Value. The publication is a report of the…

Educational Webinar: Multiple Criteria Decision Analysis for Health Care Decision Making – Emerging Good Practices
28 February 2017
On Thursday 16th March, OHE’s Nancy Devlin will lead an educational webinar, in collaboration with Kevin Marsh and Praveen Thokala, on Multiple Criteria Decision Analysis for…

New OHE Consulting Report: Exploring the Assessment and Appraisal of Regenerative Medicines and Cell Therapy Products: Is the NICE Approach Fit for Purpose?
16 February 2017
Just published is a new OHE Consulting Report entitled: Exploring the Assessment and Appraisal of Regenerative Medicines and Cell Therapy Products: Is the NICE Approach Fit…

Value and Cancer: Journal of Cancer Policy Special Issue
25 January 2017
A special issue of the Journal of Cancer Policy entitled ‘Value and Cancer’ includes three papers authored by OHE researchers.
Routine Funding in the NHS in the UK of Medicines Authorised Between 2011 and 2016 via the European Centralised Procedure
1 December 2017
The centralised procedure was created in 1995 to facilitate access to innovative medicines across the European Union. Since then the scope of authorisation via the centralised…
Antimicrobials Resistance: A Call for Multi-disciplinary Action. How Can HTA Help?
1 October 2017
This briefing provides a detailed summary of a symposium held at the HTAi 2017 meeting in Rome. This briefing provides a detailed summary of a symposium…