Unlocking the Value of Combination Therapies



A new OHE report compares the availability and access of orphan medicinal products (OMPs) in the UK, France, Germany, Italy and Spain. A new OHE Consulting Report has been published that compares the availability of and access to orphan medicinal…
A new OHE report compares the availability and access of orphan medicinal products (OMPs) in the UK, France, Germany, Italy and Spain.
A new OHE Consulting Report has been published that compares the availability of and access to orphan medicinal products (OMPs) in the UK (England, Scotland and Wales), France, Germany, Italy and Spain. The analysis quantifies the availability, access and the time (months) between the granting of the EU authorisation and the decision to recommendation/reimbursement in each country.
The European Commission’s Orphan Medicinal Products Regulation, implemented in 2000, intended to incentivise the research, development and marketing of new treatments for rare and chronically disabling or life-threatening diseases. Marketing authorisation granted to OMPs, however, is only the first step; patients have access to medicines once reimbursement or health technology assessment (HTA) decisions are implemented by national health systems.
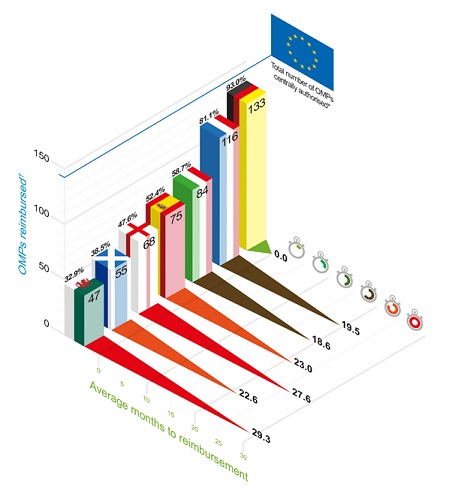
Our analysis found that since the implementation of the OMP Regulation, 143 OMPs have obtained marketing authorisation in the EU.
These OMPs are most widely accessible in Germany and France. In the other countries between 30% and 60% of OMPs are reimbursed. In England, less than 50% of OMPs are routinely funded by the NHS, with one-third of these recommended by NICE. The remaining products are directly procured and made available to patients by NHS England via commissioning policies or through the Cancer Drugs Fund.
In Germany reimbursement is automatically granted to all medicines which receive a marketing authorisation, immediately after it. For the other countries, the shortest time from authorisation to a reimbursement decision is observed in France and Italy which takes on average 19 months (see Figure 1).
Figure 1: Average months to reimbursement of OMPs

* 143 OMPs obtained a marketing authorisation since the implementation of the EU Regulation on Orphan Medicines (Regulation (EC) No 141/2000).
† OMPs reimbursed refers to Health Technology Assessment (HTA) recommendations to use or inclusion in reimbursement lists in respective national health systems.
In summary, the intended effect of the Orphan Medicinal Products Regulation – to grant equal availability to OMPs to patients in the EU – has been partially achieved, but important variations in availability and access are observed in the European countries included in our study.
Access the full OHE Consulting Report here.
This report has informed the development of a further report commissioned by Shire on equity and access to OMPs and presented at the Rare Disease Summit on 15th of March 2017 in London.
Related OHE publications include:
Garau, M. and Mestre-Ferrandiz, J. (2009). Access Mechanisms for Orphan Drugs: A Comparative Study of Selected European Countries. OHE Briefing. London: Office of Health Economics.
Mestre-Ferrandiz, J., Garau, M., O’Neill, P. and Sussex, J. (2010). Assessment of the Impact of Orphan Medicinal Products on the European Economy and Society. OHE Consulting report. London: Office of Health Economics.
For more information please contact Bernarda Zamora at OHE.
An error has occurred, please try again later.
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages.
Keeping this cookie enabled helps us to improve our website.
Please enable Strictly Necessary Cookies first so that we can save your preferences!
