Drug Development/R&D
New medicines lead to improved patient outcomes and societal benefits, but R&D is a long and costly process. Ongoing health economics research aims to find the right balance between incentivising manufacturers to innovate and ensuring affordable access for health systems.

OHE Lunchtime Seminar: The UK Biotech Sector and Brexit: Past Performance and Future Prospects
25 September 2017
OHE Lunchtime Seminar with Sir Geoffrey Owen and Professor Michael Hopkins. The biotech sector of the US has enjoyed a success that the UK sector has…

An Insurance Framework for Funding New Antibiotics
22 September 2017
A new paper sets out an insurance model as part of a global solution to incentivise R&D for new antibiotics to tackle multi-drug resistance. It offers…

Postdoctoral Position in The Economics of Life Sciences R&D
27 June 2017
The Structural Genomics Consortium Oxford, University of Oxford, in collaboration with OHE (Prof Adrian Towse) and Dr Jorge Mestre-Ferrandiz, is recruiting for a Postdoctoral Position in…
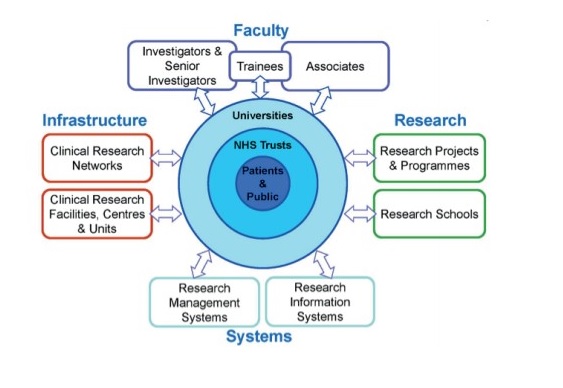
Professor Dame Sally Davies: Ten Years of the NIHR: Achievements and Challenges for the Next Decade
15 June 2017
In November 2016 Professor Dame Sally Davies delivered the 23rd OHE Annual Lecture on the topic of: Ten Years of the NIHR: Achievements and Challenges for…

Assessing the Value of New Antibiotics: Additional Elements of Value for Health Technology Assessment Decisions
30 May 2017
Without new antibiotics, more patients will die from previously treatable infections. A key issue is how the value of antibiotics can be appropriately assessed by payers…

New Publication: ‘Macro’ Evaluation of the NIHR Oxford Biomedical Research Centre
22 May 2017
OHE and RAND Europe were commissioned by the Oxford BRC to undertake a programme of top-down evaluations of aspects of the impact of the BRC. This…

Incentives for New Drugs and Vaccines to Tackle Anti-Microbial Resistance
16 May 2017
Resistance to antibiotics is growing. Additional R&D is required. Both Transferable Intellectual Property Rights and Market Entry Rewards should be explored for use in Europe as…

New Publication: Gene Therapy: Understanding the Science, Assessing the Evidence, and Paying for Value
7 March 2017
Just published is a new report entitled: Gene Therapy: Understanding the Science, Assessing the Evidence, and Paying for Value. The publication is a report of the…

Incentives for New Drugs to Tackle Anti-Microbial Resistance
1 May 2017
Resistance to antibiotics is growing, posing a major health risk in rich and poor countries. Additional ways of rewarding R&D are required. Mechanisms designed to encourage…