US

Bold politics – what about the economics?: An overview of President Trump’s ‘Most Favoured Nation’ Executive Order
2 June 2025
This is the first in a series of OHE Insights on the history, implications and possible impact of President Trump’s Executive Order on a “Most Favoured Nation” (MFN) pricing model for pharmaceuticals.

Around the World in HTAs: United States – Making HTA Great Again
11 June 2024
In this Insights series, Around the World in HTAs, we shed light on HTA around the world. In this edition, Chris Sampson and Dan Ollendorf take us to the United States.

The Inflation Reduction Act: Price negotiation underway for the first 10 drugs
29 November 2023
The first 10 drugs included in the Medicare Drug Price Negotiation Program created by the Inflation Reduction Act (IRA) were announced at the end of August. We discuss what they are, what they show us, and the potential ripple effects.

The US Inflation Reduction Act: What Do the Experts Think?
25 May 2023
In August 2022, President Biden signed the Inflation Reduction Act (IRA) into law. In this OHE Insight, Amanda Cole explores the expert discussions had at ISPOR US 2023.

New free educational program launched – Explaining the U.S. Inflation Reduction Act
9 May 2023
We have launched a free online explainer to the U.S. Inflation Reduction Act. It makes essential viewing for anyone wanting a sound knowledge of the technical detail of the Act and how it relates to pharmaceutical payment and coverage.

The World Needs New Antibiotics. A Proposed US Program to Develop Them Offers a High Pay-Off
16 November 2022
A Center for Global Development (CGD) paper authored by OHE’s Adrian Towse and CGD’s Rachel Silverman Bonnifield shows a very high expected return on investment (ROI)…

Limitations of CBO’s Simulation Model of New Drug Development as a Tool for Policymakers
1 June 2022
In recent years, U.S. policymakers have been considering reforms to tackle high and rising prescription drug spending, including unprecedented direct limits on prices and price growth…

OHE Critique of CBO’S Pharmaceutical Investment Model Provides Warning for Policymakers on Reliability of Estimates
3 December 2021
As US policymakers consider the potential implications of the drug pricing reforms contained within the Build Back Better Act, OHE releases a critique of the Congressional…
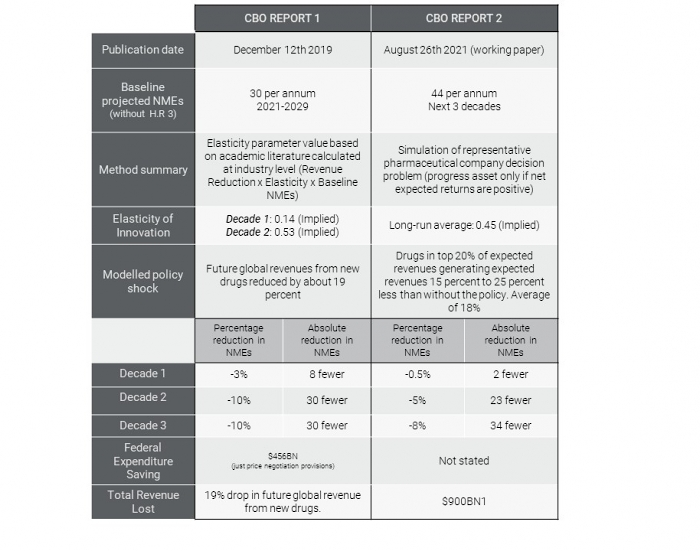
Does the New CBO Report Change Anything?
15 September 2021
Since we began our project to consult experts on the impact of H.R. 3. on pharmaceutical innovation the CBO has released a new working paper. Given…