New antibiotics are urgently needed, but their development is hindered by market failure. A ‘volume-delinked model’ subscription approach has been suggested to overcome this market failure, providing compensation regardless of sales volume.
Key takeaways
- All investors agreed that the UK proposals are hugely significant and most of them considered pull incentives to be a key mechanism for incentivising the development of novel antibiotics.
- The scheme as proposed may not be sufficiently predictable to support investment in terms of the proposed contract conditions or the assessment of eligibility and clinical value.
- The upper bands (£15m, £20m) are deemed sufficient to be effective as England’s proportion of a global pull incentive, but the lower bands (£5m, £10m) are not.
- While the UK effort in isolation will not represent an effective pull incentive, combined action from the EU and the USA would be expected to provide a sufficient minimum pull incentive. Substantial harmonisation on the target products supported across schemes will be required for global sums to add up to a sufficient incentive.
- Areas for proposed improvements include 1) extension of the initial contract length, 2) some flexibility regarding the assessment of eligibility and clinical value, 3) a transparent collaborative process for the review of scoring criteria and contract conditions over time, 4) and clarification of the long-term commitment to the scheme.
This report examines the likely success of the newly proposed pull incentive for antibiotic development in the UK by seeking the perspectives of (potential) investors in the antibiotics space. Investors are key stakeholders in the development of new antibiotics, as their buy-in is critical to unlock the required funds and expertise for the development of novel antibiotics.
Nine interviews were conducted, involving financial investors, and biopharma executives. We found that most investors were generally optimistic that a pull incentive could be a useful policy tool if it provides a sufficient monetary incentive globally (which will require high levels of consistency on eligibility and award/scoring criteria between jurisdictions) and is sufficiently predictable for investors and innovators.
While the UK effort in isolation will not represent an effective pull incentive, combined action from the EU and the USA would be expected to provide a sufficient minimum pull incentive. Substantial harmonisation on the target products supported across schemes will be required for global sums to add up to a sufficient incentive, see Figure 1.
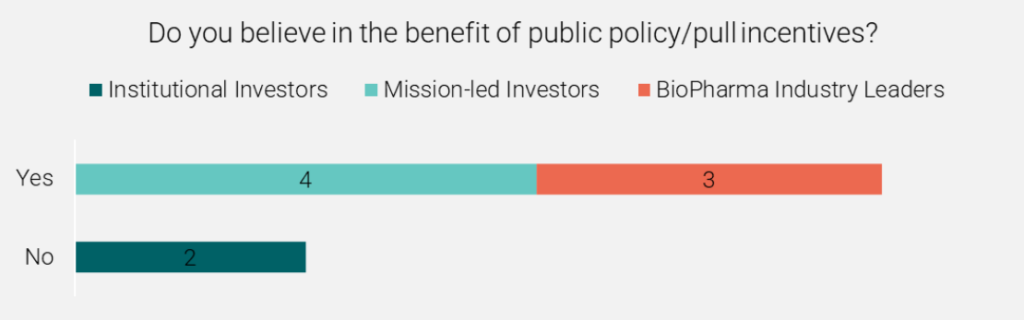
Figure 1 Investor perspective on public policy/pull incentives in AMR
The results of the interviews with investors indicate that there are several areas in which current UK proposals could be strengthened, enabling them to become a more convincing and effective policy tool for investors:
- Consider whether the initial contract length could be extended beyond three years.
- Implement a transparent collaborative process for the review of scoring criteria and contract conditions over time.
- Consider some form of early advice or dialogue to enable developers (and thus investors) to predict how their product may perform against the scoring criteria.
- Replace wide value bands with smaller more incremental value bands to mitigate the potential systematic undervaluation of eligible products.
- Clarify the long-term commitment to the scheme.
The report was commissioned and funded by The Association of the British Pharmaceutical Industry (ABPI).


