Unlocking the Value of Combination Therapies

New antibiotics are urgently needed, but their development is hindered by market failure. A ‘volume-delinked model’ subscription approach has been suggested to overcome this market failure, providing compensation regardless of sales volume. NHS England held a public consultation on proposals for a subscription style revenue guarantee to stimulate research and development (R&D) in antimicrobials from July to October 2023. Under the scheme, eligible antimicrobials will be assessed against a multicriteria scoring system and will be placed into one of four value bands that correspond with annual payment serving as a revenue guarantee.
Interviews were conducted with five leading clinical experts and five realistic dummy antibiotics were created and scored against the proposed criteria. This report contains an overview and analysis of the scoring system, and discusses how new antibiotics are likely to be valued based on the dummy product exercise.
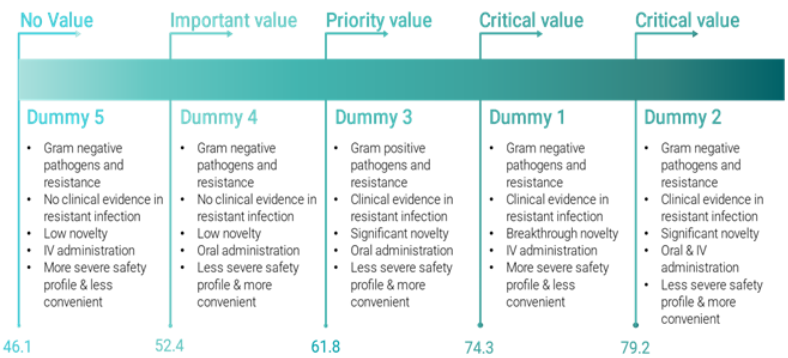
Figure 1 Overview of scoring and value of Dummy products
The results of the dummy exercise indicates that the scoring system generally rewards 1) products with broad spectrum and activity against gram-negative priority pathogens and resistance mechanisms, 2) products that are more innovative in terms of chemical structure or mechanism of action, 3) products that are administered orally, 4) products that were tested clinically in patients with resistant infections showing superiority over current standard of care, and 5) products that address an area of clinical unmet need in the UK.
The report was commissioned and funded by The Association of the British Pharmaceutical Industry (ABPI).
This report is complementary to the OHE Report “Proposals for a Novel UK Antimicrobial Subscription Model: The Investor Perspective”.
Proposals for a Novel UK Antimicrobial Subscription Model: How Will Antibiotic Innovation be Scored?
