Since 1996, in its yearly PPRS Report to Parliament, the Department of Health in England has published international price comparisons of branded medicines used in the…
Since 1996, in its yearly PPRS Report to Parliament, the Department of Health in England has published international price comparisons of branded medicines used in the primary care setting. The most recent PPRS Report contains comparisons for 2010. In a report just out, OHE Consulting updates these comparisons for the UK through 2011, using the same methodology as the Department of Health uses in its PPRS reports.
Since 1996, in its yearly PPRS Report to Parliament, the Department of Health in England has published international price comparisons of branded medicines used in the primary care setting. The most recent PPRS Report contains comparisons for 2010[1]. In a report just out, OHE Consulting updates these comparisons for the UK through 2011, using the same methodology as the Department of Health uses in its PPRS reports. The OHE Consulting analysis also examines the challenges involved in comparing medicine prices across countries.
International price comparisons are not straightforward. Many issues and factors determine how prices of medicines in the UK compare to those in the rest of the world. There is no single preferred method for analysing price differences across countries, but some methods are more appropriate than others. Choosing which to use depends in part on the objective of the comparison.
In its most recent PPRS Report to Parliament, the Department of Health included the 250 top-selling branded medicines used in primary care in England in 2011; medicines used predominantly in the hospital and home care settings were excluded. The OHE Consulting report uses these same criteria and also is based on data collected for the following countries: Australia, Austria, Belgium, England, Finland, France, Germany, Ireland, Italy, The Netherlands, Spain, Sweden and the US. To ensure the medicines included do match, various databases were used.
The total sample of preparations included in the OHE Consulting report is 799, although the sample varies by country since not all countries market all 799 preparations[2]. The sample was weighted by the volume of sales in England to show what the NHS would pay for medicines if they were priced at the levels in the comparator countries. Additional details on the methodology are explained in the report, available for download from the OHE website.
In the early 2000s, UK prices were among the highest of the comparator countries, according to Department of Health PPRS reports. In 2007-2008, the UK dropped substantially in the rankings, from mid-position to the bottom of the pricing range. Based on OHE Consulting’s analysis, prices in 2011 were still in the bottom quartile for the leading branded medicines in primary care in the UK, as the table shows.
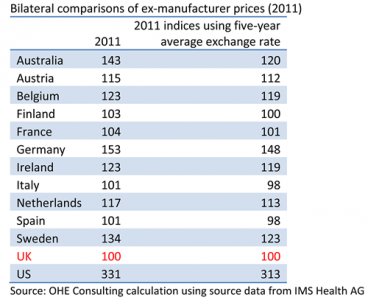
Column 2 shows the 2011 results using the average exchange rates for Q4 2011. Column 3 shows the 2011 indices using the average Q4 exchange rates for the five years 2007-2011. Indices using five-year average exchange rates, intended to smooth out the year-by-year volatility of exchange rates, also are reported in the PPRS Report to Parliament.
Download O’Neill, P., Puig-Peiró, R., Mestre-Ferrandiz, J. and Sussex, J. (2012) International comparisons of medicines prices, 2011 indices, methodology and results. Consulting Report. London: Office of Health Economics.
For further information contact Dr Jorge Mestre-Ferrandiz at OHE Consulting.
