Sign up to our newsletter Subscribe
A Spotlight on Haemophilia Therapies

Sign up to our newsletter Subscribe


World Rare Disease Day aims to raise awareness of rare diseases and the impact they can have on patients. This blog explores OHE’s contributions to research into rare diseases and orphan medicines. World Rare Disease Day is celebrated on the…
World Rare Disease Day aims to raise awareness of rare diseases and the impact they can have on patients. This blog explores OHE’s contributions to research into rare diseases and orphan medicines.
World Rare Disease Day is celebrated on the last day of February 28th or 29th in a leap year (the rarest day) to raise awareness of rare diseases and the impact they can have upon patients. The European Medicines Agency defines rare diseases as life-threatening or chronically debilitating conditions that have a prevalence of fewer than 5 cases per 10,000 population. Despite the low numbers of persons with any specific disease, rare diseases occur in up to 4% of births and 6-8% of the population in the EU; this equates to around 27-36 million people. Although rare diseases are individually rare, they are collectively common. Furthermore, 72% of rare diseases are genetic, meaning that they disproportionately affect children.
Treatments for many rare conditions include the use of medicines; these medicines are referred to as orphan medicines. New innovative treatments are continually being developed, but there are a number of economic and ethical challenges in the development and evaluation of medicines for these conditions that are distinct from those faced by medicines for more common conditions. These challenges are discussed in an OHE Consulting Report, particularly in the context of NICE’s appraisal of rare and ultra-rare conditions. We also considered to what extent standard appraisal processes are appropriate for ultra-orphan medicines, with a deep dive into NICE’s Highly Specialised Technologies pathway. Further research was conducted in this area, in which we developed case studies to describe these challenges and better understand the intricacies of the NICE appraisal process. We found a flexible and pragmatic approach towards uncertainty; these positive examples include consideration of bespoke statistical modelling, expert testimony, and/or the use of real-world evidence. Less positive examples were also observed, especially around inflexible consideration of uncertainties inherent around ultra-rare conditions.
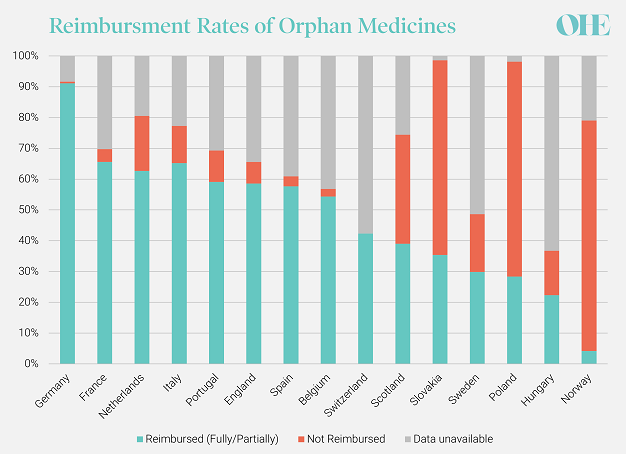
The challenges associated with evaluating orphan medicines are demonstrated by the high degree of variability in access to orphan medicines across Europe. A recent analysis by OHE explored the reimbursement rates of 215 orphan medicines in 15 European countries from the introduction of Regulation No 141/2000 on Orphan Medicines in 2000 to the end of 2019. We observed substantial variation in the levels and speed of national reimbursement of orphan medicines. We found that Germany had the highest level of coverage, with 91% of orphan medicines being reimbursed. The three countries with the lowest reimbursement rate were Poland, Hungary and Norway (all below 30%).

OHE has also carried out research into the role of pharmaceutical innovation in the development of orphan medicines. In collaboration with EFPIA, we developed case studies that illustrate the specific drivers and barriers in developing medicines for rare diseases. We found that innovation requires hard work, sustained investment, partnership between innovators and regulators, and often, a bit of luck to unlock patient benefit.
We recently hosted a Masterclass exploring innovation for small populations, tackling the issues of generating the right incentives to stimulate research and development into new therapeutic solutions for rare diseases whilst also managing concerns around health system affordability. The Masterclass will soon be available on-demand via the OHE website, where you can download the presentations from OHE experts and other industry thought-leaders on the topic. Email events@ohe.org for further information.
An error has occurred, please try again later.
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages.
Keeping this cookie enabled helps us to improve our website.
Please enable Strictly Necessary Cookies first so that we can save your preferences!



