In this insight, commissioned and funded by Chiesi S.p.A, we reflect on the discussions that took place at ISPOR Europe 2023 and how environmental impact is factored into HTA.
With healthcare systems responsible for 4%-5% of global greenhouse gas emissions (Watts et al., 2017), it is imperative that we scrutinise the environmental impact of the pharmaceutical industry, acknowledging its crucial role in shaping a sustainable future. Two panel sessions at ISPOR Europe 2023 in Copenhagen discussed this topic. These sessions explored whether environmental impact should be embedded into health technology assessments (HTA).
In this insight, we highlight key points from each speaker and dissect how the panellists believe we could move towards the collective vision for a greener, healthier future.
The four panellists in the first session on Monday, 13th November, were:
- Jens Grueger (Moderator) – Partner and Director at Boston Consulting Group.
- Sean D. Sullivan – Professor of Health Economics at the CHOICE Institute, University of Washington School of Pharmacy.
- Meindert Boysen – Head of International Affairs at the National Institute for Health and Care Excellence (NICE).
- Alessandra Madoni – Head of Global Respiratory Value and Access at Chiesi Group.
The four panellists in the second session, held on Tuesday 14th November, were:
- Grace Hampson (Moderator) – Associate Director at the Office of Health Economics.
- Nadine Henderson – Senior Economist at the Office of Health Economics.
- Saskia Knies – Coordinating Advisor at the National Health Care Institute (ZIN).
- Isabell Crasto de Stefano – Global Public Affairs Manager AIR at Chiesi Group.
Setting the scene
Jens Greuger kicked off the first session with an overview of how the conversation on environmental sustainability has changed over the last 50 years. He highlighted how a holistic understanding of the complex relationship between the environment, society and the government has emerged in recent decades to drive conversations around the interactions between health, health systems and the environment.
Jens also presented original research suggesting that payers expect the likelihood of sustainability playing a role in drug access / pricing / reimbursement / procurement to increase in the next five years (see Figure 1). This is aligned with HTA perspectives given in both sessions that HTA agencies are interested in this space and are exploring whether or how such considerations should be included in their processes.
Figure 1: Payer expectations on the likelihood of sustainability playing a role in pricing and reimbursement
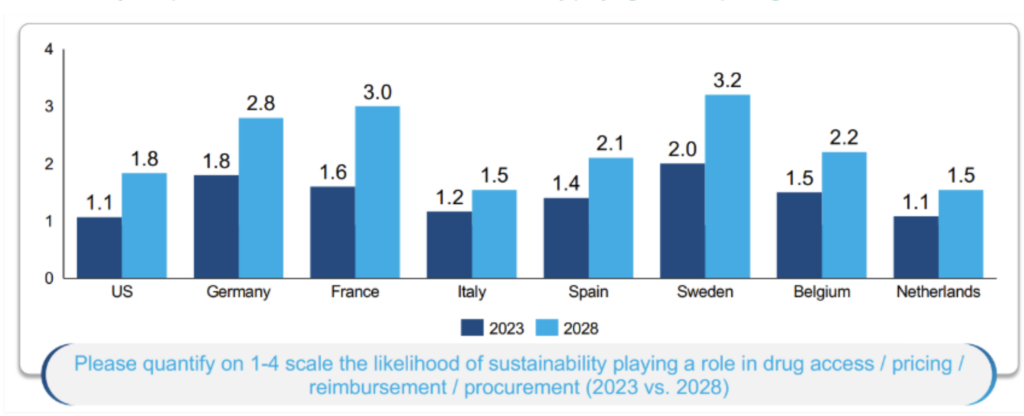
Note: figures represent the average score assigned by experts.
Source: BCG research.
Grace Hampson opened the second session by presenting findings from OHE research into the contributions of healthcare to national carbon footprints across 13 countries, with estimates in 2015 ranging from 2.3% in Italy to 10.7% in Russia, and the US sitting at 6.7%. Moreover, in the UK, it is estimated that the manufacture, supply, and use of pharmaceuticals represent around a quarter of the NHS’s total carbon footprint. Clearly, for countries and health systems to achieve ambitious Net Zero targets, changes across the pharmaceutical industry will be critical.
Both introductions demonstrated that emissions and other factors contributing to environmental sustainability arise across the pharmaceutical value chain, with emissions concentrated early in the supply chain, as shown in Figure 2. A combination of challenges, such as complex, long, global supply chains; the highly regulated nature of the industry in terms of product safety, consistency, and efficacy; and the high proportion of product wastage, puts the pharmaceutical industry in a unique position. This means that targeted research and bespoke solutions are needed.
Figure 2: Sources of Emissions Across the Supply Chain
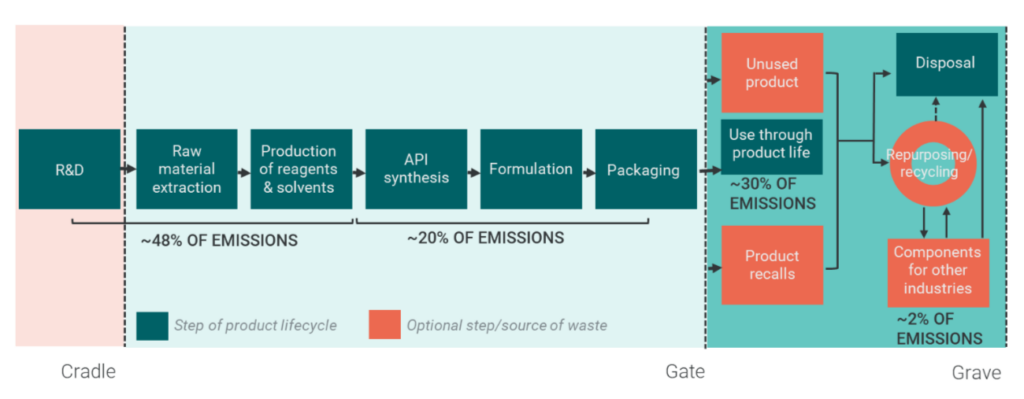
Sources: Firth et al., 2022 – adapted from High Value Manufacturing (HVM) Catapult sustainability report representing approximate emissions across the pharmaceutical value chain industry-wide (unpublished).
The pharmaceutical industry needs adequate incentives to prioritise improving environmental sustainability. Sean Sullivan began his presentation by delving into five sustainability incentives in the pharmaceutical industry: government regulation, tax incentives, grants and funding, public perception, and market access.
He provided examples of where these have been used in practice (see Table 1) and examples of where pharmaceutical companies have taken actions to become more sustainable (see Table 2). These examples show that many pharmaceutical companies are interested in engaging in more sustainable practices, with some companies also looking to establish financial instruments, such as Pfizer’s Sustainability Bond. Companies are also interested in translating their efforts into economic impact and valuing their environmental efforts, including their impact beyond carbon offsets.
Source: adapted from content presented at the session by Sean D. Sullivan.
Source: adapted from content presented at the session by Sean D. Sullivan.
The final part of Sean’s presentation focused on how HTA organisations could consider environmental sustainability in reimbursing and pricing pharmaceuticals. Table 3 provides a list of suggestions made. In his discussion, Sean emphasised that the ISPOR value flower should also be updated to consider environmental effects.
Table 3: Suggestions for how HTA organisations may take account of environmental sustainability in their assessment of pharmaceuticals
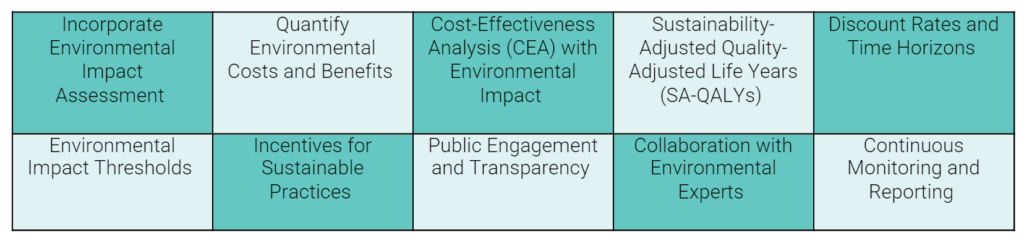
Source: adapted from content presented at the session by Sean D. Sullivan.
Nadine Henderson also outlined various types of incentives which could be employed, broadly aligning with those listed above. Nadine presented some of the current incentives and commitments being adopted, focusing on India’s Net Zero roadmap – highlighting the importance of this due to the high volume of pharmaceutical manufacturing in India.
She also commented that Scandinavian countries are leading across the board in terms of sustainability. For example, in Norway, the Norwegian Hospital Procurement Trust (Sykehusinnkjøp HF) introduced criteria to reduce the environmental impact of pharmaceutical products in 2019, and from 2020 to 2022, they launched eight pharmaceutical procurement processes with environmental requirements attached.
Nadine argued that progress towards decarbonisation is gaining momentum and that we must continue accelerating the pace. This means moving beyond quick fixes to answer the long-term questions which require more thought. These include determining if environmental impacts should be considered in HTA or reimbursement processes and the practical hurdles to implementing this.
Nadine also raised that there could be unintended consequences from pursuing an assessment of environmental impact. For example, any health traded for lower carbon emissions may be disproportionately given up by lower-income individuals with lower health. Therefore, the wealth-health gradient could potentially be exacerbated through a more environmentally-centred HTA process, raising equity concerns. Consideration must therefore be given to distributional concerns when designing any such amendments.
HTA Perspective
Meindert Boyson set out how NICE (HTA Agency for England) is approaching the incorporation of environmental sustainability.
As a starting point, just by doing its ‘normal’ work, i.e., helping practitioners and commissioners get the best care to patients while ensuring value for the taxpayer, NICE can already help achieve reduced environmental impacts alongside improved patient outcomes. This aligns benefits for both the patient and the environment and does not require any ‘trade-offs’ to be made. However, if trade-offs are required, participants in workshops conducted by NICE felt that health outcomes should always be prioritised.
Looking to the future, a recent paper by colleagues at NICE identified four potential options for how NICE could more explicitly consider environmental impact in decision-making: 1) information conduit, 2) environment-focused evaluation and full evaluation alongside clinical and cost outcomes with a 3) parallel environmental evaluation 4) integrated environmental evaluation.
However, the challenges lie in putting robust methods in place, determining which data should be collected, how it should be collected, and incorporating this into the chosen methodology. Meindert highlighted that research was ongoing to find solutions to these questions but also raised two additional questions: 1. whether the required skills exist in HTA bodies and industry to interpret environmental data and determine impact, and 2. whether it is proportionate to incorporate environmental impact in ‘all’ HTAs of pharmaceuticals considering the capacity constraints in agencies and industry.
Saskia Knies discussed environmental impact from the Dutch HTA perspective. Saskia summarised progress in this area by stating that environmental impact is not yet included in HTA as the methods still need further development. Methods are needed to assess how environmental impact analysis should be combined and how to include environmental impact analysis in HTA.
She highlighted that we are at the very start of this journey, with these methods still needing refinement and reliable data on the environmental impact of health technologies not yet widely available. However, there are case studies of the assessment of the environmental impact of some individual technologies.
At ZIN, they recognised a GREEN Team in June 2022 with a mandate to incorporate sustainability in their mission and tasks of the organisation and manage their internal emissions. In planning how they could incorporate environmental impact on the products assessed via HTA, they proposed a four-step growth model to determine what may be possible, as shown in Figure 3. At present, the idea at ZIN is not to have environmental sustainability included in cost-effectiveness analysis, but for it to be used as an additional criterion in assessments. This aligns with how budget impact and disease severity are currently considered by ZIN.
Figure 3: Growth model for determining what is possible for including environmental impact IN HTA related products assessed by ZIN

Source: adapted from content presented at the session by Saskia Knies.
Industry Perspective
Alessandra Madoni and Isabell Crasto de Stefano both highlighted the commitment by Chiesi to ensure that sustainability principles are embedded in product design and permeate the entire company.
The contribution of pressurised Metered-Dose Inhalers (pMDIs) to global warming was a theme across both presentations. Chiesi is investing more than €350 million to develop and industrialise low-carbon pMDIs that will reduce the carbon footprint of pMDI inhalers by up to 90%. This is borne from Chiesi’s commitment to positively impact the planet and provide a much-needed therapeutic option for patients that addresses the main source of the environmental footprint of the company.
In Alessandra’s presentation, she also talked about how the movement towards sustainability can maintain momentum. She highlighted that the value of sustainable practices should be shared and that, to seize opportunities, companies should monitor and respond to public sentiment, proactively address any negative issues, and invest in R&D and technology to deal with medical, societal and environmental unmet needs. Also, permanent engagement with employees and other stakeholders is needed to foster and grow a culture of shared value.
Isabell discussed how global efforts will be required to effect change, echoing Alessandra’s call for the collaboration of different stakeholders across the value chain. She also pointed out that short-term decisions on sustainability impact long-term profitability for many companies in the industry.
She raised concerns relating to the numerous ambitious targets being set by many pharmaceutical companies, questioning whether these targets are achievable and whether there is a risk of green-washing practices.
Finally, she stressed that patients need to be at the centre of policy designs around sustainability. Importantly, policies and healthcare solutions should not undermine the treatment options for patients. We need to take a holistic view of the patient pathway, considering the impact of environmental policies at each stage on the redesigned patient journey. This will help us ascertain the full impact on both patients and the environment.
Audience Polling
Polls were put to the audience across both sessions. Of 122 respondents in the first session, 77% reported that environmental sustainability is not currently sufficiently reflected in HTA value assessment frameworks and should be included more formally. The majority (52%, N=125) also supported incentives such as extended exclusivity / data protection, priority review vouchers, and / or tax relief for the company over allowing a higher price at launch (26%) or simply rejecting any products that negatively impact the environment (18%).
In the second session, respondents were asked to signal the extent to which they consider it is a) appropriate and b) reasonable for environmental impact to be incorporated into HTA. 74% of 72 respondents felt that it is either somewhat appropriate or very appropriate, while 66% (N=75) felt it is somewhat feasible or very feasible. These results show reasonable agreement across the audiences from the two sessions, with both audiences also demonstrating an appetite for exploring the emerging challenges via the Q&A.
The Lively Q&A Sessions
Both sessions concluded with challenging and thought-provoking questions and discussion with the audience. In the first session, questions focused on how we can identify products where environmental impact is particularly crucial, and the additional expertise needed in this evolving space. Concerns about greenwashing and the importance of agreed-upon standards to mitigate risks were also raised. In the second session, the focus was on expanding the discussion beyond health economics with a need to avoid narrowly defining solutions and encouraging instead a broader perspective encompassing environmental economics. The importance of intergenerational equity was also discussed, and the audience and panellists all encouraged consideration of the consequences of the impact of climate change on the health of future generations.
Conclusion
The collective insights from the panellists weave a narrative of progress, challenges, and commitment within the healthcare industry’s journey towards sustainability. As we navigate this complex intersection of health and the environment, the industry’s collaborative efforts, innovative solutions, and commitment to change offer the potential for a greener and healthier future for all. However, there are still many unanswered questions, and the panellists agree that further research will be needed to figure out the future of environmental impact within HTA.
To bring together people interested in sustainability, Grace is working with ISPOR to explore the appetite for, and potential remit of, a special interest group on this topic. To learn more about sustainability and be part of considering how some of the questions discussed in the panel sessions could be answered, please email sigs@ispor.org indicating your interest.
References
Watts, N. et al. (2019) ‘The 2019 report of The lancet countdown on health and climate change: Ensuring that the health of a child born today is not defined by a changing climate’, The Lancet, 394(10211), pp. 1836–1878. doi:10.1016/s0140-6736(19)32596-6.
Firth, I., Hitch, J., Henderson, N. and Cookson, G., 2022. Supporting the Era of Green Pharmaceuticals in the UK – OHE. [online] OHE – Leading intellectual authority on global health economics. Available at: https://www.ohe.org/publications/supporting-the-era-of-green-pharmaceuticals-in-the-uk/ [Accessed 5 Dec. 2023].
UK-CHI-2300780 I January 2024



