Unlocking the Value of Combination Therapies



Europe already has some experience with the impact of biosimilars on pricing, including a price war in Germany for EPOs. Whether that experience will be repeated, however, depends on a number of factors, including the originator’s pricing strategy and reservations…
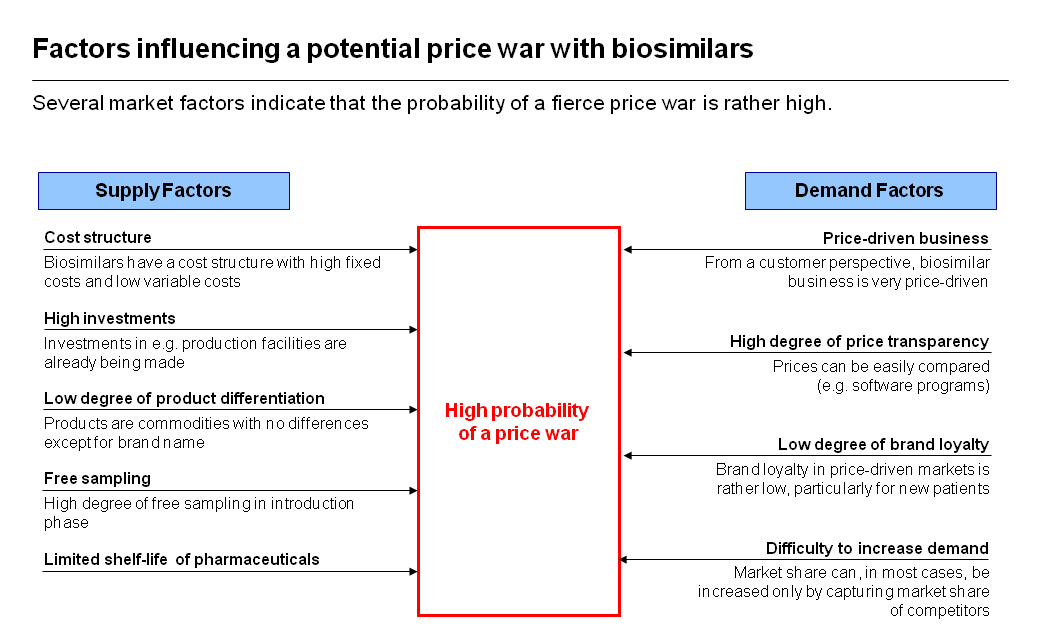
Europe already has some experience with the impact of biosimilars on pricing, including a price war in Germany for EPOs. Whether that experience will be repeated, however, depends on a number of factors, including the originator’s pricing strategy and reservations by payers and physicians about potential risks.
This is the sixth in our series of posts based on the OHE seminar and summarises the remarks of Dr Matthias Liefner of Simon Kucher and Partners.
In Europe, pricing of biosimilars already has begun to affect the market for biologics. For example, France’s compulsory price discounts for generic drugs have been applied to EPO and somatropin biosimilars; the ensuing mandatory price reductions for the originator in the French system have made prices roughly equivalent. In German, EPO and somatropin are included in the reference price system. Quotas and guidelines enforce the use of biosimilars regionally and tenders are likely to be applied to biologics in future. In the UK, where the Primary Care Trusts decide formularies, price is expected to be an important factor.
Across Europe, however, the focus of payers on price is moderated by concerns — particularly among physicians — about the safety and side-effect profiles of biosimilars. The relative weight of price versus potential risk may vary with the disease area targeted by the biologic, however. For example, since growth hormone is given primary to children and effects are visible only over the longer term, tolerance of risk and reduced efficacy is lower. EPO and insulin, in comparison, are primarily adult medications and their effects are visible immediately. Payers are more likely to support the use of biosimilars in such cases.
The potential effect of price controls on biologics markets is illustrated by EPO in Germany. A cascade of price reductions followed the creation of a fixed reference price (FRP) group in 2007 and the entry of three biosimilars later that year – at 30% below the originator price. A price reduction by the originator was followed by further price reductions by the biosimilars and then again by one of the originators.
Dr Liefner described what factors could determine whether price wars appear in other biosimilars markets. These are summarised in the graphic. He concluded that the extent to which these dynamics operate depends in part on the originator’s pricing strategy and on reservations, by payers and physicians, about risks – a situation likely to change as experience increases comfort with biosimilars. 
Source: Simon Kucher and Partners (click graphic to enlarge)
Dr Matthias Liefner is a Director in the Life Sciences Division at Simon Kucher and Partners.
Publication now available for download: Mattison, N., Mestre-Ferrandiz, J. and Towse, A. eds (2010) Biosimilars: How much entry and price competition will result? London: Office of Health Economics.
An error has occurred, please try again later.
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages.
Keeping this cookie enabled helps us to improve our website.
Please enable Strictly Necessary Cookies first so that we can save your preferences!
