A new OHE Consulting Report considers the data capabilities in the UK for monitoring medicine use according to indication using real world data.
In the UK and across the world, the routine collection of healthcare data is expanding, which is enhancing opportunities for research into the use and impact of healthcare services and interventions. Whilst important advances in data systems are continually being made, information gaps remain. One such gap is the ability to monitor the use of medicines according to what the medicine has been used for (the ‘indication’) when medicines have multiple indications or are used by more than one distinct patient sub-group.
Assessing the use of innovative medicines in the UK population is important for identifying variation in access, supporting the Pharmaceutical Price Regulation Scheme (PPRS), analysing the value of medicines in routine practice, and monitoring the implementation of guidance from bodies such as the National Institute for Health and Care Excellence (NICE). These activities are impeded if medicine use cannot be ascribed to indication. For example, multi-indication medicines were left out altogether from reports by the Health and Social Care Information Centre (HSCIC) in England on the Use of NICE appraised medicines in the NHS (now replaced by the Innovation Scorecard). As well as supporting efforts to assess uptake and compliance, the demand by decision-makers and regulators for ‘real world data’ (data collected outside the context of a clinical trial) is rising, in response to increased reliance on performance-based reimbursement arrangements and adaptive approaches to licensing. This signifies increased evidence requirements in the post-marketing phase, which must be supported by good quality data collection alongside routine clinical practice.
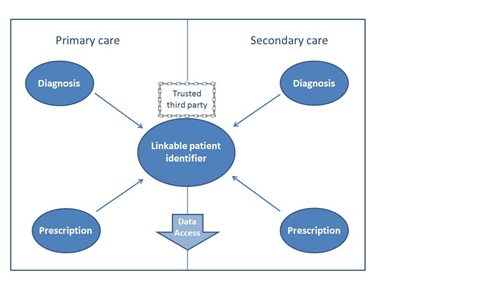
A recent OHE study, commissioned by The Association of the British Pharmaceutical Industry (ABPI), summarises current capabilities for monitoring medicine use by indication across the four countries of the UK, highlighting best practice and outlining the further steps required to improve capabilities. The report found that, whilst in no country are prescriptions routinely issued with an attached diagnosis, the data linkage capabilities to infer this are the most advanced in England. However, although the key pieces of information are collected and the required infrastructure to link these data exists, coverage of this linkable information is limited and therefore any research must rely on sample data; see Figure 1 for the required pieces of information. Whilst coverage for primary care data in Wales is wide (around 70% of the population), the key piece of missing information is prescription data in secondary care. In Scotland and Northern Ireland, hospital prescription data is similarly limited, along with linkable diagnostic data in the primary care setting.
Figure 1 Necessary information for data linkage
By assessing national data collection and access for research, as well as investigating promising local initiatives, the report makes a series of recommendations. In the first instance, hospital e-prescribing and electronic patient records must be adopted across the whole of the UK for real improvements to ensue, and centralised collection and management of that data would further facilitate linkage possibilities. In recognition of the important uses of the data for uptake evaluation and research, there should be improved access to data within the appropriate arrangements for governance.
Access the full report
here.