OHE delivered a webinar on pharmaceutical sustainability, supported by ABPI, bringing together leading experts and professionals from government, healthcare, and industry. This webinar builds on a recently published OHE report on sustainability in the pharmaceutical industry.
The impact of healthcare delivery on climate change
As our planet faces an unprecedented threat from climate change, it’s time for us to rethink and redesign our healthcare systems to be sustainable, resilient, and fit for the future.
The healthcare system accounts for around 5% of the UK’s carbon emissions, and NHS England has estimated that the manufacture, supply, and use of pharmaceuticals account for 25% of the NHS’s total carbon footprint.
How can we make the delivery of healthcare more sustainable?
To answer this question, OHE delivered a webinar, supported by ABPI, bringing together leading experts and professionals from government, healthcare, and industry. This webinar builds on a recently published OHE report.
‘Supporting the Era of Green Pharmaceuticals in the UK’ outlines the key challenges faced by the pharmaceutical industry in its efforts towards sustainability, highlighting high-priority activities that could solve the challenges presented by the climate crisis.
High refinement of products, complex supply chains, resource-intensive manufacturing and R&D, the need for constant innovation, and a lack of incentives for sustainability are barriers to achieving net-zero carbon emissions in the sector.
Pharmaceutical companies need to target interventions at the main sources of emissions in their supply chain, primarily raw material extraction, active ingredient manufacture, and energy-intensive processes, to reach Net Zero.
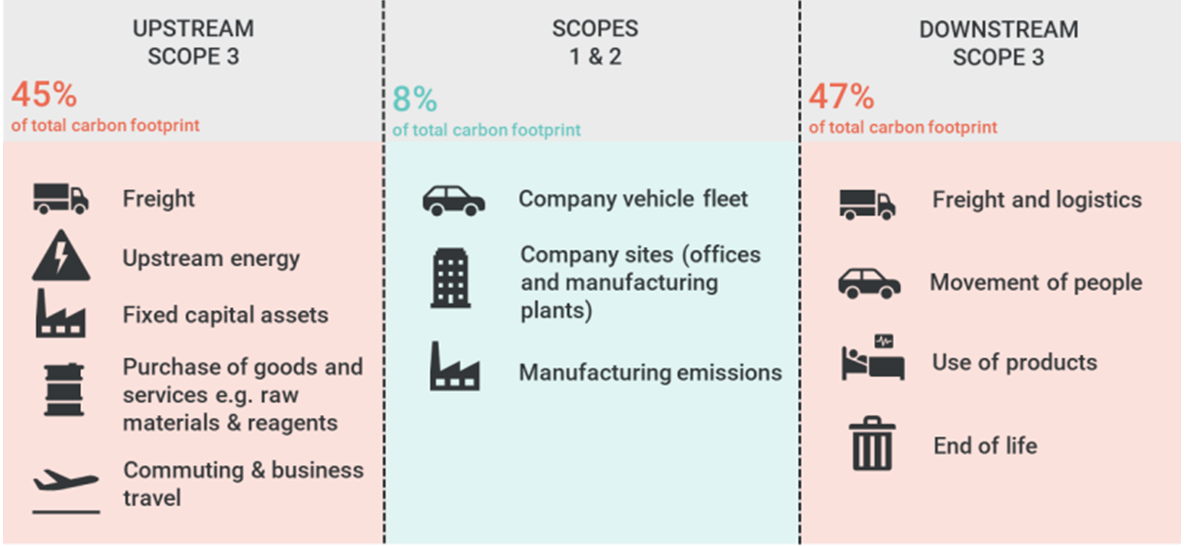
Source: GSK value chain carbon footprint
While Scope 1 and 2 emissions, caused directly and indirectly by the companies, are relatively low, Scope 3 emissions, linked to upstream and downstream factors like transportation and disposal, make up 80-90% of their carbon footprint and are beyond direct control.
To combat this, collaborations between the pharmaceutical industry, the NHS, and the UK government are integral.
OHE’s key recommendations:
- The UK government needs to invest in grid decarbonisation, implement common regulatory standards, and support the NHS’s sustainability initiatives.
- The NHS should focus on sustainable procurement processes, waste reduction, and innovation partnerships.
- The pharmaceutical industry should disclose emissions, invest in lifecycle analysis of products, and improve energy efficiency.
How are key stakeholders taking action?
The Scottish Government:
Our first panellist was Alifia Chakera, Head of Pharmaceutical Sustainability at the Health Infrastructure & Sustainability Division of the Scottish Government. She explained some of the measures the Scottish Government is taking to promote sustainability and reduce emissions in their healthcare system.
These include plans to transition to a circular model of care, prioritising the reuse, repurposing, and remanufacturing of materials, as well as developing a green formulary that considers the environmental impact of pharmaceutical products, aiming for value-based and environmentally friendly healthcare options.
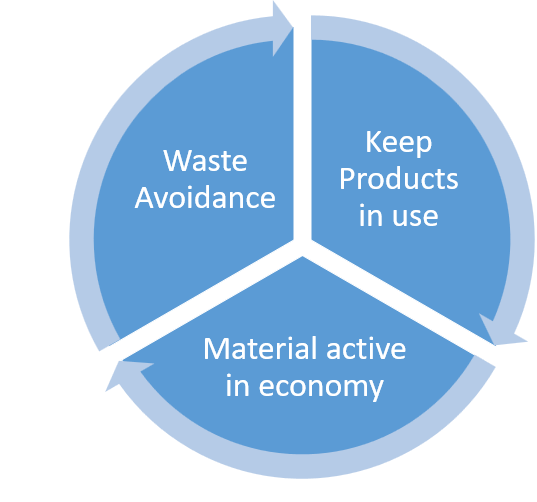
Source: Wendy Rayner
Another significant initiative is the National Green Theatre Program (NGTP). The impetus of this program is taken from NHS Highlands, which employed a multidisciplinary approach to implementing 11 projects, demonstrating not only reductions in carbon emissions but also significant cost savings of £130,000 per year – a positive sign for the financial viability of implementing sustainability changes. The NGTP has identified over 5O sustainability projects that they plan to test, assess and roll out nationally.
These successes have been the result of collaboration with university partners, sustainable healthcare coalitions, and organisations like the Scottish Health Technology Group; these collaborations, involving multiple stakeholders, facilitate the exchange of ideas, experiences, and best practices, allowing for a more comprehensive and informed approach to decision-making.
AstraZeneca:
From industry, we heard from Matt Shaughnessy, Head of Operations Sustainability at AstraZeneca and Sustainability Lead at the Medicines Manufacturing Industry Partnership.
He explains the work that AstraZeneca is performing in placing environmental protection at the core of their sustainability strategy, including commitments to transitioning to an electric vehicle fleet, transitioning out inhaler propellants, and reducing scope 1 and 2 greenhouse gas emissions by 98% by 2026. In addition, AstraZeneca will halve its scope 3 emissions and become carbon negative by 2030 on a pathway to science-based net zero by 2045 at the latest.
While these are important goals for the company, Matt also stresses a move away from focusing only on their own business, recognising the importance of working with partners upstream and downstream in the supply chain. This includes identifying suppliers that most significantly contribute to emissions, educating and setting expectations on the reporting of key science-based metrics and working with them to ensure they commit to new requirements.
It is also important to partner with other companies in the industry, aligning on standards through the Sustainable Markets Initiative and Pharmaceutical Supply Chain Initiative, and providing practical resources to make renewable energy affordable.
NICE:
Our third and final panellist was Koonal Shah, Associate Director of Science Policy and Research at the National Institute for Health and Care Excellence (NICE). Koonal clarified NICE’s motivations and approach towards sustainability. Considering the OHE report, Koonal reflected on the ways in which environmental data can be used in economic evaluation.
Koonal explained that NICE’s approach to improving healthcare sustainability is to support the NHS by ensuring that any care provided is necessary and of high quality, developing guidance to reduce unnecessary care and avoidable harm from care that is necessary.
While the NICE Board recently supported the continued use of a health sector perspective in NICE’s reference case for health economic assessments, as opposed to a systems approach or a societal perspective, there are considerations as to how to include environmental data and ecological impact in economic evaluation. Presently, however, there is a lack of consensus, common methods, and unbiased comparable analyses for incorporating environmental information into the evaluation process.
There are hopes that in the medium term, data will become available as industry stakeholders begin to routinely collect emissions data, though questions for HTA agencies like NICE remain regarding what data to collect, when and how to collect it, as well as precisely what to do with such data.
Insights and Agreements from the Panel Q&A Session
Following presentations from each of our expert panellists, we held an audience Q&A, leading to an insightful discussion on topics including incentivising sustainability in the sector, challenges around innovation and lifecycle assessment, as well as the opportunities for consensus building presented by such challenges.
Some of the key points of agreement for further action raised in the discussion included the recognition that to build environmental considerations into Health Technology Assessment, a collaborative effort from stakeholders is necessary to develop standardised methods for assessing greenhouse gas emissions and the ecological impact of healthcare delivery.
Panellists agreed that there are sustainability incentives for pharmaceutical companies from shareholders and employees, as environmental responsibility is seen as key to attracting talent and ensuring profitability. Although there are challenges, including the need for rapid market access, sustainability can lead to efficiency gains and cost savings. Producing environmentally friendly, clinically- and cost-effective medicines can provide a competitive market advantage.
Significant challenges remain in the shift towards green infrastructure, particularly with the NHS facing financial pressures; however, through harnessing efficiency savings and long-term planning (avoiding the ‘mad march syndrome’), investments in sustainability that may have higher upfront costs but lead to savings over time delivering a green dividend, sustainable efficiency gains can help to offset financial pressures.
As demonstrated by the examples given by our panellists, it is clear that the greatest advances towards sustainability in the healthcare sector have been made through partnerships and collaborations.
Points for further discussion
Insights from our diverse panel of experts highlighted not only the successes and challenges inherent within actions towards sustainability but also prompt new questions and areas of discussion, including:
- Implementing environmental impact data into evaluations:
What are the most effective methods for collecting and standardising environmental emissions data in Health Technology Assessments? And is it feasible to expand our data inclusion beyond carbon emissions to consider additional environmental impacts, such as ecotoxicity, tied to our medical practices?
- Interdisciplinary collaboration and partnerships:
How can we expand collaboration among stakeholders, reaching beyond the healthcare sector to include governmental departments, environmental agencies, and biological and environmental sciences, to capture a wider range of environmental effects beyond carbon emissions?
- Addressing Scope 3 Emissions:
What strategies can pharmaceutical companies implement to reduce Scope 3 emissions, which are largely beyond their direct control? Can successful collaborations offer insights into facing these challenges and implementing effective measures?
Increased dialogue and alignment between key stakeholders will allow us to answer these questions and tackling these challenges present great opportunities to ensure the continued innovation, research, and action required to improve the sustainability of healthcare delivery into the future.
Key opportunities for the UK
- Methodological advances in carbon emission data collection and use within economic evaluation, in international collaboration between NICE and peer HTA bodies like CADTH
- The development of a world-leading set of standards through the British Standards Institute
- Partnership and collaboration between the key stakeholders in the healthcare sector, fostering continued, innovative research.
To this end, as part of our commitment to evidence-based healthcare, OHE continues to conduct research on sustainability and welcomes partnerships and collaboration in this area. If you are interested in exploring this topic area further please contact cashton@ohe.org as we work to unite global industry representatives, regulators, payers, and academics to address critical global health challenges in this area.
Thank you to our experts for taking part and sharing their insights on this important subject. In a rapidly changing world, healthcare’s role in climate action has never been more critical. We invite you to join us in this ongoing conversation and collaborative effort as we continue to explore new research and solutions that will shape a greener, more sustainable future for healthcare. Together, we can turn the challenges of today into the opportunities of tomorrow.
Watch our Sustainability in the Era of Green Pharmaceuticals Webinar On-Demand
In this 90-minute webinar, we built on the results of the “ Supporting the Era of Green Pharmaceuticals in the UK” report. Consequent recommendations for industry, health systems and Governments as outlined in the report were explored with our expert panel.

