Unlocking the Value of Combination Therapies



In this blog, we reflect on some of the key learnings from the recent pull and push strategies to develop COVID-19 vaccines in the UK, the US, and the European Union. We use our recent work on portfolio management, pricing,…
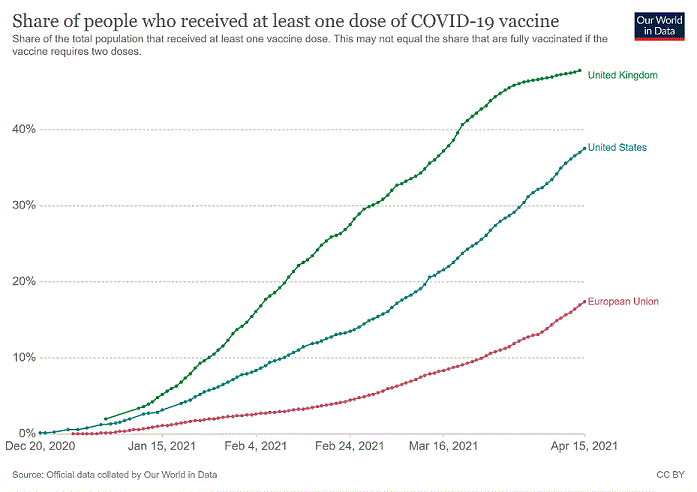
In this blog, we reflect on some of the key learnings from the recent pull and push strategies to develop COVID-19 vaccines in the UK, the US, and the European Union. We use our recent work on portfolio management, pricing, and procurement of COVID-19 vaccines (forthcoming as an OHE report) to organise the review.
In this blog, we reflect on some of the key learnings from the recent pull and push strategies to develop COVID-19 vaccines in the UK, the US, and the European Union. We use our recent work on portfolio management, pricing, and procurement of COVID-19 vaccines (forthcoming as an OHE report) to organise the review.
After the shock caused by the first wave of COVID-19, discovering vaccines against the virus and administering them quickly to the population became the utmost priority worldwide, especially once subsequent waves of COVID-19 were inevitable. Non-medical interventions such as lockdowns and social distancing to prevent the spread of the disease and the collapse of healthcare systems translated into unprecedented falls in gross domestic product. Vaccines became a critical tool not only to save lives but also to ensure that those alive could conserve their livelihoods. Yet, while many of us spent most of 2020 following the number of deaths associated with COVID-19, we started 2021 confronted with the stunning disparity of the COVID-19 vaccination rollout within OECD countries. As of April 14, 2021, the UK has administered 47.79 doses per 100 population, the US 37.05, while the EU lagged at 16.94. The difference in the vaccination rollout between the UK and the US on the one hand, and the EU on the other, have been the subject of many articles and commentaries [a,b,g].

To understand how this problem could be overcome, we were commissioned by a European Parliamentary Group (Renew Europe) to investigate ‘Key factors on how to procure, pay for, distribute, and use vaccines for COVID-19: A European perspective’. In that piece, we discussed tactics for optimal procurement, pricing, and distribution of COVID-19 vaccines, as of November 2020, focusing on portfolio management and rollout strategies. This blog summarises the report’s recommendations and reflects on possible factors behind the slower pace of rollout of the EU vaccination programme.
In the report, we advocate for a vaccines portfolio management strategy that incentivises vaccine candidates’ simultaneous development to achieve vaccine-induced herd immunity as fast as possible. We also suggested that candidates in the portfolio be classified in two priority levels, with homogenous tiers in terms of vaccine development stage, probability of success, and vaccine type (i.e. viral vector, mRNA, proteinic subunit, or inactivated SARS-COV-2).
We also advise that advanced purchase agreements (APA) are a tool to stimulate rapid scaling up of production capacity. For this purpose, we suggest that the expected (and later, observed) quality of vaccines should steer the negotiation of volumes, prices, and delivery timeframes.
In terms of processes, we recommend creating an EU Steering Committee (SC) to design and oversee the process of contracting vaccines based on a banded system. The banded system would consider a pre-defined Target Product Profile(1) (TPP), including vaccine efficacy and safety and immunogenicity, ease of delivery and storage, dose regimen, and durability of protection. The SC should ensure that the European Commission, the European Medicines Agency, and the Member States’ (MSs) healthcare agencies coordinate via the European Network for Health Technology Assessment (EuNetHTA), so that vaccine prices relate to their value, including their extended value-added elements (d). Value-based pricing would be especially beneficial after the initial urgent round of vaccine procurement, whose rapid development and roll out might otherwise be at risk.
We advise that the SC should establish a robust monitoring and pharmacovigilance system to update data on vaccines in the pipeline and those already rolled out. Over time, production capacity should be shifted from lower to higher-value vaccines according to new information and MSs’ unmet needs, as appropriate. The MSs should also develop strategies to avoid overreacting to very rare or ultra-rare safety issues, which are to be expected if only from the sheer volume of the population vaccinated in a short amount of time.
In the report, we also provide some general recommendations for allocating vaccines to MSs and distributing vaccines within MSs, advocating for the guiding principles of effectiveness and solidarity. For that purpose, considering factors beyond population size would be advisable, i.e., the pressure of COVID-19 on the country’s healthcare system and the economy, the MS’s infrastructure, etc. The distribution of vaccines within each MS should prioritise vulnerable groups and key workers, and be flexible to accommodate individual levels of mobility and the availability of each vaccine type.
Finally, we discuss the need to develop a targeted public communication strategy about benefits, risks, and the importance of achieving high vaccination rates to stop COVID-19 deaths, reach vaccine-induced herd immunity, restore the healthcare systems’ capacity to serve all who need care, and rebuild the economy. Information campaigns should understand and responsibly use social media and mass media communication tools to connect with populations unsure about vaccination and monitor the campaigns’ effectiveness to fine-tune them.
Since the report’s launch on November 20, 2020, the European Commission’s executive team has implemented some of the recommended strategies. There have been negotiations with different vaccine producers simultaneously. APAs have been used to guarantee rapid scaling up of production capacity. Most countries have developed a plan for vaccine delivery.
However, we have identified areas in which the proposed tactics have not been applied due to inaction or unforeseen events. These may partly explain the slower rollout of vaccination in the EU. We discuss a list of them below:
In retrospect, our suggestion of tactical negotiations of contracts with another Tier 2 protein subunit would have been beneficial. Novavax, a protein subunit vaccine we classed in Tier 2, is currently under review by EMA, and negotiations are only ongoing now [e,f]. A two-tiered plan and negotiations starting before the end of 2020 would have been preferable to rushing it in 2021.
The vaccination rollout puzzle has been subject to intense debate producing multiple views and explanations. In sum, despite its incommensurable devastating effects, COVID-19 has taught the world valuable lessons such as the need for pandemic and catastrophes preparedness and the necessity for innovating regulatory strategies to achieve medical innovations at an extraordinary pace. The ultimate message has probably been how much humans value health and the lengths they will go to re-establish it when threatened.
(1) See the WHO TPP for Covid-19 vaccines for an example: https://www.who.int/publications/m/item/who-target-product-profiles-for-covid-19-vaccines
References
[a] Covid: Why is EU’s vaccine rollout so slow? – BBC News
[b] Why the EU’s covid-19 vaccination programme went wrong? The Commission should have done much better. The Economist. Briefing, April 3, 2021, Edition.
[c] Why has the EU been so slow to roll out a Covid vaccination programme? | Bruegel by Guntram Wolff, Director of the Brussels-based Bruegel think tank.
[d] The Broader Value of Existing Vaccines in the Fight Against COVID-19: Beware of Tunnel Vision | OHE
[e] EMA starts rolling review of Novavax’s COVID-19 vaccine (NVX-CoV2373), February 3, 2021.
[f] EU close to vaccine supply deal with Novavax February 11, 2021.
[g] What Covid vaccines cost – and the countries paying over the odds. The Week. March 30, 2021.
[h] Vaccines: A Very European Disaster – P. Krugman. The New York Times. March 18, 2021.
[i] Commission approves second contract with Moderna
[j] Timeline of EU action | European Commission
[k] COVID-19 vaccines: development, evaluation, approval and monitoring
[l] Covid vaccines: Will drug companies make bumper profits? BBC Business, December 18, 2020
[m] Funding and manufacturing boost for UK vaccine programme – GOV.UK (www.gov.uk)
[n] Covid-19 Deals Tracker: More Than 9.60 Billion Doses Reserved Worldwide (bloomberg.com)
[o] AstraZeneca’s COVID-19 vaccine authorised for emergency supply in the UK
An error has occurred, please try again later.
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages.
Keeping this cookie enabled helps us to improve our website.
Please enable Strictly Necessary Cookies first so that we can save your preferences!



