It is estimated that one in three people born in 2015 in the UK may develop dementia, and clinical development success rates for dementia drugs are consistently lower than those for other therapy areas.
A new analysis conducted by OHE’s Dr Fraser Lewis estimates that one in three people born in 2015 in the UK will develop dementia. A second OHE analysis, conducted by Grace Marsden and Dr Jorge Mestre-Ferrandiz, looks at dementia treatments currently in clinical development, and reveals that clinical development success rates for dementia drugs are consistently lower than those for other therapy areas (a finding consistent with other similar analyses done to date).
The estimates of future cases of dementia are a result of work commissioned by Alzheimer’s Research UK. The estimates were based on three components:
- estimates of how many people born in 2015 will survive to a given age;
- age and sex specific estimates of the incidence of dementia;
- an epidemiological model capable of measuring the flow of people between non-dementia and dementia, while mimicking overall population dynamics consistent with survival data.
Data was collected from the Office for National Statistics (ONS) and the Cognitive Function & Ageing Study (CFAS I), and an epidemiological model was built.
The model calculated that:
- 27% of males born in 2015 in the UK will develop dementia;
- 37% of females born in 2015 in the UK will develop dementia;
- 32% of people born in 2015 in the UK will develop dementia.
These estimates are based on conservative assumptions (for example that life expectancy will not improve in the future) and only consider late onset dementia, and therefore could be an underestimate of the total number of people who will get dementia.
Access the press release from Alzheimer’s Research UK here.
Read the story on the NHS choices website here.
Download the full OHE analysis here.
The research on drug development success rates was part of an R&D landscape and pipeline analysis of the dementia drug development arena. The analysis was based on clinical trial registries and a pipeline database. This work was undertaken in collaboration with a Clinical and Technical Expert Group (CTEG) chaired by Professor Lefkos Middleton, Imperial College London, in support of the Dementia Integrated Development Initiative, a global programme led by the UK Government to improve diagnosis and treatment of dementia worldwide.
The analysis identified approximately 2,000 trials for dementia treatments (based on the European Union Clinical Trials Register and the U.S. National Institutes of Health database (October 2014/January 2015)). Of these 2,000 trials, 129 had been suspended, withdrawn, or terminated early, but only 45% of the 129 provided a reason for this. See Figure 1 for the explanations given.
Figure 1: Reasons for termination/suspension/withdrawal of trials (n=129)
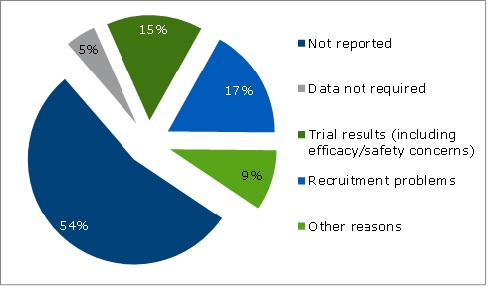
Source: OHE analysis from IMS LifeCycle R&D Focus database (October/November 2014), and input from CTEG.
The analysis also identified 900 different products for dementia indications, 197 of which were in “active” development, and only 17 of which were marketed (based on IMS LifeCycle R&D Focus database (October/November 2014), and input from CTEG).
The majority of the products in active development were classified as disease modifying (66%) rather than symptom modifying (31%), but this split did not carry through to the marketed treatments. In fact only one marketed disease modifying drug was identified; idebenone. This drug is marketed for treatment of cognitive defect within a relatively small range of countries.
Analysis of the dementia pipeline amongst other therapy areas showed that 4% of drugs in the discovery phase across all therapy areas have dementia listed as the lead indication. This proportion reduces to 1% at phase III, and 0.5% of those that are marketed.
Phase success rate calculations revealed that dementia indications have lower success rates than other therapy areas (see Figure 2).
Figure 2: Phase success probabilities
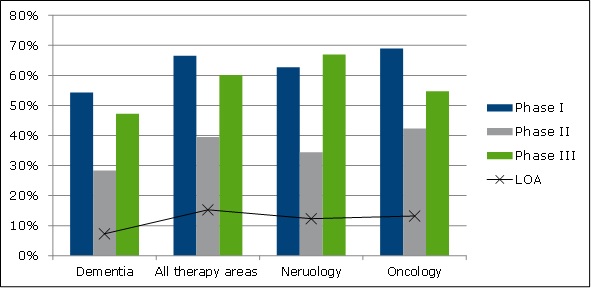
LOA = Likelihood of approval. Calculated as the probability of being marketed from phase I.
Source: OHE analysis from IMS LifeCycle R&D Focus database (October/November 2014), and input from CTEG & Hay et al., 2014. Clinical development success rates for investigational drugs. Nature Biotechnology, 32(1), pp.40–51.
The likelihood of being marketed from phase 1 = 7.3% for dementia, and 15.3% for all therapy areas.
Access a preliminary report from the Dementia Integrated Development Initiative here.
Reference for OHE analysis: Marsden, G. & Mestre-Ferrandiz, J., forthcoming. Dementia: the R&D Landscape. OHE Research Report. London: Office of Health Economics.