Health Policy and Regulation

Plan For A New EU Pharmaceutical Legislation: What Will It Mean For Pharmaceutical Innovation?
12 April 2024
The European Commission and European Parliament have set out their proposals for a reformed EU pharmaceutical legislation which could substantially change the incentive ecosystem for the first time in 20 years.

The Dynamics of Drug Shortages
9 January 2024
Drug shortages are an industry-wide problem. Numerous factors may be considered as contributing to drug shortages across the globe. In this report, we discuss the global issue of drug shortages, summarise the main reasons for shortages as presented in the literature and our quantitative analysis. Finally, we provide recommendations for policy makers.

The Inflation Reduction Act: Price negotiation underway for the first 10 drugs
29 November 2023
The first 10 drugs included in the Medicare Drug Price Negotiation Program created by the Inflation Reduction Act (IRA) were announced at the end of August. We discuss what they are, what they show us, and the potential ripple effects.

The Benefits of Early Engagement with Payers and Patient Representatives: The Case Study of a MoCA Pilot Project on ANCA-associated Vasculitis
30 June 2022
OHE and Vifor Pharma present a poster at the last European Conference on Rare Diseases (ECRD) on the Mechanism of Coordinated Access to orphan medicinal products…

Ignoring the needs of our future workforce could have disastrous consequences for the UK NHS and its patients.
12 May 2022
Ignoring the needs of our future workforce could have disastrous consequences for the UK NHS and its patients. In economics, we have a pretty complicated name…

How Does Pharmaceutical Spending Drive Innovation in Europe?
7 February 2022
A new publication by Kourouklis and Gandjour (2022) investigates the impact of pharmaceutical spending on domestic early-stage pharmaceutical innovation in Europe. They find that European spending…

Limitations of CBO’s Simulation Model of New Drug Development as a Tool for Policymakers
1 June 2022
In recent years, U.S. policymakers have been considering reforms to tackle high and rising prescription drug spending, including unprecedented direct limits on prices and price growth…

OHE Critique of CBO’S Pharmaceutical Investment Model Provides Warning for Policymakers on Reliability of Estimates
3 December 2021
As US policymakers consider the potential implications of the drug pricing reforms contained within the Build Back Better Act, OHE releases a critique of the Congressional…
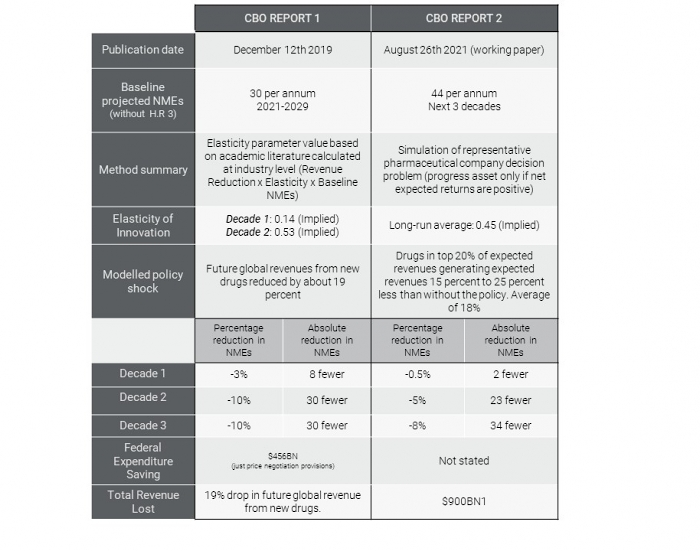
Does the New CBO Report Change Anything?
15 September 2021
Since we began our project to consult experts on the impact of H.R. 3. on pharmaceutical innovation the CBO has released a new working paper. Given…