This blog was commissioned by Janssen: Pharmaceutical Companies of Johnson & Johnson.
Discounting is a standard procedure for adjusting the value of costs and benefits for the time at which they occur in health economic evaluations. Gene therapies pose fresh challenges for discounting practice by health technology assessment (HTA) agencies. Within this blog, we provide an overview of discounting in health economic evaluations and discuss why discounting is an important issue for gene therapies, using a potential gene therapy for X-Linked Retinitis Pigmentosa (XLRP) as an example. We conclude that there are at least three discounting methods which could address the main issues, but more research is needed to compare the challenges and effects of implementing each of them in HTA and therefore which is most appropriate in which circumstances.
Why do we discount costs and effects?
Would you prefer to receive £100 today or £100 next year? For several reasons, most people would choose £100 today. This is because people typically prefer to enjoy things sooner rather than later, and there is always a chance that something will happen between now and next year which would prevent them from enjoying the £100.
There is also an opportunity cost associated with spending money today. By delaying £100 of spending today and investing that money in a financial asset offering a positive risk-free real rate of return, more money will be available to spend next year (in real terms).
These principles apply not only to the future costs but also to the future health effects associated with an intervention. This is because healthcare transforms money and other resources into health. As a result, an intervention which generates health equivalent to the value of £100 this year will be prioritised over an intervention which generates health equivalent to the value of £100 next year.
For these reasons, HTA agencies apply a technical correction called discounting in economic evaluations, which involves applying lower weights to costs and health effects that are expected to occur in the future.
How do HTA agencies discount future costs and effects?
Most countries recommend that the same discount rate is applied to costs and effects (uniform discounting) and that the rate should not change over time, i.e., the same rate should be applied to costs and effects regardless of how far into the future they occur. Nevertheless, there is significant variation in the discount rates recommended by national HTA agencies around the world (see Figure 1). A small minority of countries recommend a lower rate for effects than costs, including Belgium1, the Netherlands2, and Poland3.
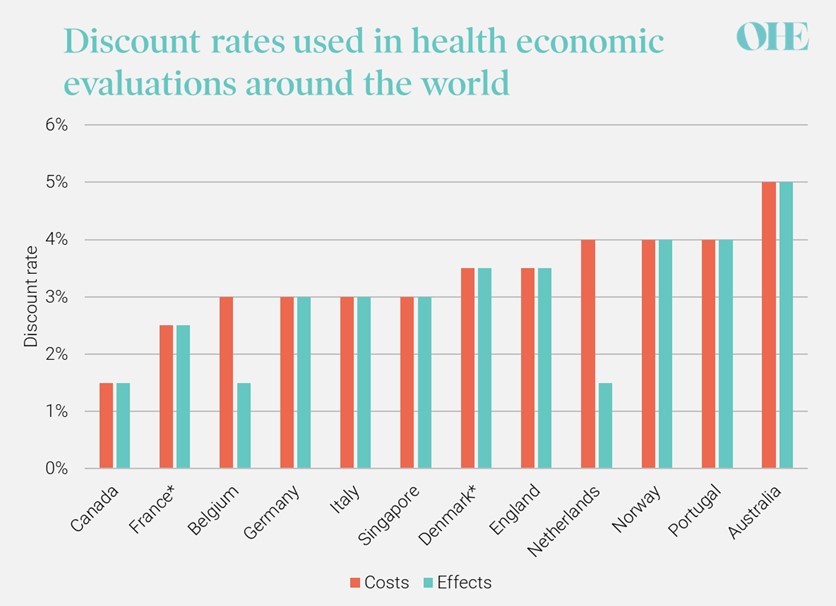
FIGURE 1: DISCOUNT RATES IN NATIONAL GUIDELINES FOR HEALTH ECONOMIC EVALUATIONS
Sources: Canada4, France5, Belgium1, Germany6, Italy7, Singapore8, Denmark9, England10, Netherlands2, Norway11, Portugal12, Australia13
Notes: in countries marked *, the discount rate declines over time meaning that costs and health effects expected to be realised after a certain number of years are discounted at a lower rate (30 years in France and 35 years in Denmark).
Why is discounting so relevant for future gene therapies?
Discounting can affect an economic evaluation’s incremental cost-effectiveness ratio (ICER) to various degrees. For some health technologies, notably those that are a one-off treatment, one pays at the time of intervention while reaping the benefits in later years. In such cases, the ICER is computed based on undiscounted intervention costs but often highly discounted future benefits (e.g. QALYs gained).
A potential gene therapy targeting XLRP may be one of the most extreme examples of a technology where discounting will be highly impactful. XLRP is a form of inherited retinal disease (IRD) that progresses very slowly until a person is considered blind, typically in their forties14. The gene therapy would likely be administered as a one-off injection during the person’s teenage years. Yet, the main health effect – preventing blindness – only occurs much later in life due to the slow-progressing nature of the condition14. Assuming the treatment benefit is durable and does not require re-treatment, and in the absence of a managed entry agreement addressing one-off costs, the intervention costs would be incurred fully today, while the benefits would gradually accrue over a lifetime.
Compelling evidence exists, demonstrating just how sensitive the cost-effectiveness of gene therapies is to discount rates. Internal analysis of a hypothetical therapy for XLRP shows that a discount rate of 3.5% per year for costs and effects (the current practice by NICE10) results in an ICER of $88,445.Raising it to 5% (current practice in Australia, for example15) increases the ICER by 87% to $165,042 (see Figure 2). Similarly, based on the economic model in the Luxturna submission to ZIN in the Netherlands, raising the discount rate for effects from 1.5% to 4% (and keeping the discount rate for costs at 4%) is found to increase the ICER by 81%16.
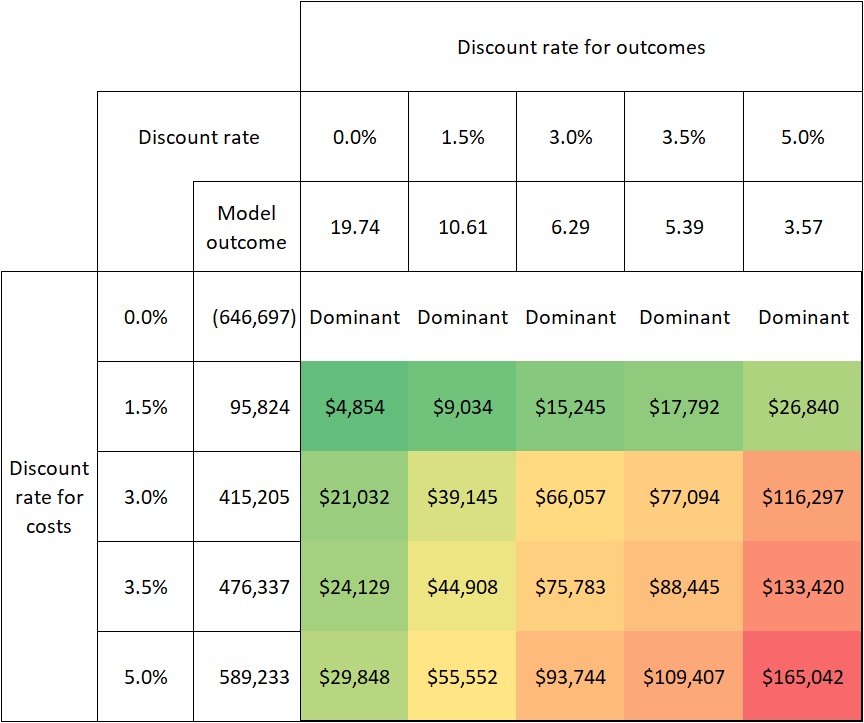
FIGURE 2: SENSITIVITY OF COST-EFFECTIVENESS TO DISCOUNT RATES IN XLRP
Source: pharmaceutical company analysis of a hypothetical gene therapy for XLRP
For gene therapies, the choice of discount rate and discounting approach has a strong influence on cost-effectiveness. Therefore, discounting will affect the likelihood of gene therapies receiving reimbursement and approval and might impact patients’ access to such therapies.
How can we improve discounting of gene therapies?
Possible approaches to overcoming the challenges posed by current discounting practices in the value assessment of gene therapies include adjusting the discount rates for costs and effects for specific technologies, using a lower rate for effects than costs (differential discounting), applying a lower discount rate to long-term effects (time-variant discounting), or some combination of these. Below we will discuss three particular opportunities using a gene therapy targeting XLRP as an example: 1) using lower discount rates for costs and effects, 2) differential discounting, and 3) time-variant discounting.
Opportunity 1: use lower discount rates for gene therapies
The first opportunity is to apply a lower discount rate to gene therapies than standard health technologies. NICE recommends departing from the standard rate of 3.5% for costs and effects to 1.5% if the technology under review meets the three following conditions10:
- The technology is for people who would otherwise die or have a very severely impaired life
- It is likely to restore them to full or near-full health
- The benefits are likely to be sustained over a very long period
Some gene therapies may meet all three conditions. For example, this lower discount rate was applied in NICE’s assessment of Zolgensma for spinal muscular atrophy (SMA)17. However, it is unclear whether a gene therapy targeting XLRP would meet all three of these conditions. For example, in condition 1 it is unclear what is meant by “very severely impaired” – XLRP has no direct effect on mortality but causes severe vision loss and therefore does have a considerable negative impact on health-related quality of life. In addition, conditions 2 & 3 are hard to prove in the medium term, given the specific challenges in demonstrating the potentially life-long clinical effects of gene therapies.
It is important to note that many countries recommend conducting sensitivity analysis around the discount rate for any health technology. For example, NICE recommends exploring a discount rate of 1.5%10, Germany recommends exploring discount rates of 0%-5%6, and Austria recommends exploring discount rates as high as 10% per year18. Regardless of the discount rates chosen for these sensitivity analyses, it is critical that decision-makers consider their results as important additional information in their deliberations.
Opportunity 2: use a lower discount rate for effects than costs
Another potential solution is to apply a lower discount rate to effects than costs (differential discounting) to all health technologies or to gene therapies and similar technologies. This solution would improve the cost-effectiveness of gene therapies because it would result in more weight being applied to future benefits compared to current uniform discounting approaches.
Academic literature supports a lower discount rate for health than for costs19–22. This approach is commonly justified on the grounds that the consumption value of health does not decrease over time as wealth grows, and therefore applying the same wealth effect to health as to costs is inappropriate. Differential discounting has also been discussed explicitly in the context of gene therapies23–25. Drummond et al. (2019)24 for example, argue that analysts should explore differential rates for gene therapies but do not believe there is not a strong enough case to depart from uniform discounting generally.
The Netherlands, Belgium, and Poland all currently recommend differential discounting. Interestingly and inconsistently, NICE recommends a reference case discount rate of 3.5% for both costs and effects in England10, while HM Treasury recommends discounting costs at 3.5% per year and health benefits at 1.5% per year for the first 30 years, as detailed in the Green Book 26.
Opportunity 3: lower the discount rate over time
As well as setting different discount rates for costs and effects, a third possibility is to reduce rates over time, e.g., apply a lower discount to costs and effects that occur beyond a certain number of years after the intervention. For example, HM Treasury recommends discounting costs and effects beyond 30 years at a lower rate26, as does France5. In France, HAS recommends a discount rate of 2.5% for costs and effects occurring in the first 30 years after the intervention, decreasing to 1.5% after 30 years. The Danish Medicines Council stipulates that the Danish Ministry of Finance’s estimate of the socioeconomic discount rate should be used for costs and QALYs9. The Danish Ministry of Finance recommends a form of time-variant discounting – 3.5% for the first 35 years, 2.5% for years 36-70, and 1.5% for year 70 onward27. This form of discounting may also be more appropriate for gene therapies as well as other technologies whose health effects arise mostly in the future while costs are incurred in the short term.
Outlook – Is there a “cure” for short-sighted discounting approaches of gene therapies targeting inherited eye disease like XLRP?
Gene therapies often come with high upfront costs, while their health effects and other benefits may not fully be realised for many years. Therefore, their estimated cost-effectiveness is highly dependent on the discount rate(s) used. Setting a discount rate that is too high will disproportionately disadvantage gene therapies, and uniform discounting may be inappropriate for technologies with a significant lag between costs and benefits. Applying these forms of discounting to gene therapies could delay or prevent patients from accessing potentially transformative therapies.
The discounting challenge is not unique to gene therapies. Other health technologies like cell therapies, vaccines, and other preventive interventions also share this feature. Therefore, HTA agencies must reconsider their discounting guidelines where these are currently not fit for purpose. At a minimum, sensitivity analyses should be used to explore the impact of different discounting scenarios on the overall result. HTA agencies need to revisit their discount rates and ensure they are fit-for-purpose. What is needed is a compromise between two objectives, namely ensuring consistency across health technologies without unduly “punishing” those technologies that deliver lifetime effects.
Opportunity 1 may be the simplest way of ensuring that gene therapies are not undervalued in economic evaluations. However, it will create an inconsistency in methods because it involves applying one discount rate to most interventions and another to gene therapies. On the other hand, opportunities 2 and 3 will ensure consistency in discounting practice across technologies but depending on how the rates are set there is a chance that gene therapies will be under- or overly favoured compared to traditional technologies. The latter may be the case if the discount rate for effects is too low compared to the discount rate for costs under opportunity 2.
All three opportunities can potentially address the challenges posed by gene therapies, but their success will depend on the quality of the data used to set levels for the discount rate. Depending on the estimation method, this may include macroeconomic data, data on societal time preferences and government borrowing costs. The success of these strategies will also depend on the weights placed on scenario and sensitivity analyses in deliberative decision-making. HTA agencies should carefully consider the sensitivity of the results to the discount rate and to the discounting method in deliberative decision-making. The emphasis on deliberative decision-making varies across HTA settings. It should be an essential part of the HTA process for gene therapies though, given the unanswered question of how best to value their future costs and effects in economic evaluations.
References
- Cleemput I, Neyt M. Belgian guidelines for economic evaluations and budget impact analyses: second edition. Published online 2012. https://www.kce.fgov.be/en/publications/all-reports/belgian-guidelines-for-economic-evaluations-and-budget-impact-analyses-second-edition
- Zorginstituut Nederland (ZIN). Guideline for economic evaluations in healthcare. Published online 2016. Accessed October 24, 2022. https://english.zorginstituutnederland.nl/publications/reports/2016/06/16/guideline-for-economic-evaluations-in-healthcare
- Agencja Oceny Technologii Medycznych i Taryfikacji (AOTMiT). Wytyczne oceny technologii medycznych (machine translation). Published online 2016. Accessed January 20, 2023. https://www.aotm.gov.pl/wytyczne-oceny-technologii-medycznych/
- Canadian Agency for Drugs and Technologies in Health (CADTH). Guidelines for the Economic Evaluation of Health Technologies: Canada 4th Edition. Published online 2018. https://www.cadth.ca/guidelines-economic-evaluation-health-technologies-canada-0
- Haute Autorité de Santé (HAS). Choices in Methods for Economic Evaluation (English version). Published online 2020. https://www.has-sante.fr/upload/docs/application/pdf/2020-11/methodological_guidance_2020_-choices_in_methods_for_economic_evaluation.pdf
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswese (IQWiG). General Methods – Version 6.1. Published online 2022. https://www.iqwig.de/en/about-us/methods/methods-paper/
- Italian Medicines Agency. Linea guida per la compilazione del Dossier a supporto della domanda di rimborsabilità e prezzo di un medicinale – Estratto sezione E (machine translation). Published online 2020. https://www.aifa.gov.it/en/linea-guida-capitolo-9
- Agency for Care Effectiveness (ACE). Drug and Vaccine Evaluation Methods and Process Guide. Published online June 2021. Accessed September 30, 2022. https://www.ace-hta.gov.sg/docs/default-source/process-methods/ace-drug-and-vaccine-evaluation-methods-and-process-guide-(june-2021).pdf
- Danish Medicines Council. The Danish Medicines Council Methods Guide For Assessing New Pharmaceuticals. Published online 2021. Accessed November 9, 2022. https://medicinraadet.dk/media/wq0dxny2/the_danish_medicines_council_methods_guide_for_assessing_new_pharmaceuticals_version_1-2_adlegacy.pdf
- National Institute for Health and Care Excellence. NICE health technology evaluations: the manual. Published online January 31, 2022. https://www.nice.org.uk/process/pmg36/chapter/introduction-to-health-technology-evaluation
- Norwegian Medicines Agency. Guidelines for the submission of documentation for single technology assessment (STA) of pharmaceuticals. Published online 2018. https://legemiddelverket.no/Documents/English/Public%20funding%20and%20pricing/Documentation%20for%20STA/Guidelines%20151018.pdf
- National Authority of Medicines and Health Products (INFARMED). Orientações Metodológicas para Estudos de Avaliação Económica de Tecnologias de Saúde (machine translation). Published online 2019. Accessed November 28, 2022. https://www.infarmed.pt/documents/15786/1431404/Orienta%C3%A7%C3%B5es+Metodol%C3%B3gicas+para+Estudos+de+Avalia%C3%A7%C3%A3o+Econ%C3%B3mica+de+Medicamentos/78d35a18-92a6-8fc4-5fde-24dab1968669
- Pharmaceutical Benefits Advisory Committee (PBAC). Guidelines for preparing a submission to the Pharmaceutical Benefits Advisory Committee Version 5.0. Published online 2016. https://pbac.pbs.gov.au/content/information/files/pbac-guidelines-version-5.pdf
- Tsang SH, Sharma T. X-linked Retinitis Pigmentosa. In: Atlas of Inherited Retinal Diseases. 1st ed. Advances in Experimental Medicine and Biology. Springer Cham; 2018:31-35. Accessed October 11, 2022. https://link.springer.com/book/10.1007/978-3-319-95046-4
- PBAC. Guidelines for preparing a submission to the Pharmaceutical Benefits Advisory Committee Version 5.0. Published online 2016. https://pbac.pbs.gov.au/content/information/files/pbac-guidelines-version-5.pdf
- Huygens SA, Versteegh MM, Vegter S, Schouten LJ, Kanters TA. Methodological Challenges in the Economic Evaluation of a Gene Therapy for RPE65-Mediated Inherited Retinal Disease: The Value of Vision. Pharmacoeconomics. 2021;39(4):383-397. doi:10.1007/s40273-021-01003-y
- National Institute for Health and Care Excellence. Onasemnogene abeparvovec for treating spinal muscular atrophy. Published online July 7, 2021. Accessed December 5, 2022. https://www.nice.org.uk/guidance/hst15
- Ludwig Boltzmann Institut B. Methodenhandbuch für Health Technology Assessment Version 1 .2012. Published online 2012. https://aihta.at/uploads/tableTool/UllCmsPage/gallery/Methodenhandbuch.pdf
- Claxton K, Paulden M, Gravelle H, Brouwer W, Culyer AJ. Discounting and decision making in the economic evaluation of health-care technologies. Health economics. 2011;20(1):2-15.
- Attema AE, Brouwer WBF, Claxton K. Discounting in Economic Evaluations. Pharmacoeconomics. 2018;36(7):745-758. doi:10.1007/s40273-018-0672-z
- Oliver A. A normative perspective on discounting health outcomes. J Health Serv Res Policy. 2013;18(3):186-189. doi:10.1177/1355819613485671
- Paulden M, O’Mahony JF, McCabe C. Discounting the Recommendations of the Second Panel on Cost-Effectiveness in Health and Medicine. PharmacoEconomics. 2017;35(1):5-13. doi:10.1007/s40273-016-0482-0
- ten Ham RMT, Klungel OH, Leufkens HGM, Frederix GWJ. A Review of Methodological Considerations for Economic Evaluations of Gene Therapies and Their Application in Literature. Value in Health. 2020;23(9):1268-1280. doi:10.1016/j.jval.2020.04.1833
- Drummond MF, Neumann PJ, Sullivan SD, et al. Analytic Considerations in Applying a General Economic Evaluation Reference Case to Gene Therapy. Value Health. 2019;22(6):661-668. doi:10.1016/j.jval.2019.03.012
- Jönsson B, Hampson G, Michaels J, Towse A, von der Schulenburg JMG, Wong O. Advanced therapy medicinal products and health technology assessment principles and practices for value-based and sustainable healthcare. Eur J Health Econ. 2019;20(3):427-438. doi:10.1007/s10198-018-1007-x
- HM Treasury. The Green Book: Central Government Guidance on Appraisal and Evaluation. Published online 2022. https://www.gov.uk/government/publications/the-green-book-appraisal-and-evaluation-in-central-governent/the-green-book-2020
- Danish Ministry of Finance. Dokumentationsnotat – den samfundsøkonomiske diskonteringsrente (machine translation). Published online January 7, 2021. https://fm.dk/media/18371/dokumentationsnotat-for-den-samfundsoekonomiske-diskonteringsrente_7-januar-2021.pdf
Related research
- Besley, S., Henderson, N., Towse, A. & Cole, A. (2022) Health Technology Assessment of Gene Therapies: Are Our Methods Fit for Purpose?. OHE Consulting Report. Available from https://www.ohe.org/publications/health-technology-assessment-gene-therapies-are-our-methods-fit-purpose.
- Brassel, S. and Steuten, L. (2020a) The Broader Value of Vaccines: The Return on Investment From a Governmental Perspective. OHE Consulting Report. Available from https://www.ohe.org/publications/broader-value-vaccines-return-investment-governmental-perspective.
- Brassel, S. and Steuten, L. (2020b) Are Discount Rates Used in UK Vaccine Economic Evaluations Jeopardising Investment in Immunisation Programmes? OHE Blog Post. Available from https://www.ohe.org/news/are-discount-rates-used-uk-vaccine-economic-evaluations-jeopardising-investment-immunisation.